44 limiting reagent and percent yield worksheet
DOCX Limiting Reactant and % Yield Worksheet EXTRA PRACTICE: Limiting Reactant and Percent Yield Worksheet. Chlorine can replace bromine in bromide compounds forming a chloride compound and elemental bromine. The following equation is an example of the reaction: 2KBr(aq) + Cl. 2 (aq) 2KCl(aq) + Br 2 (l)(a)When 0.855g of Cl. 2 and 3.205g of KBr are mixed in solution, which is the limiting ... Limiting Reactant Worksheet Answers - Limiting Reactant ... What was the percent yield? Limiting reactants worksheet # 2 . C) calculate the number of moles of the excess reagent remaining at the end of. Determine the number of grams of excess reagent left. How many moles of nh3 can be produced from the . Write the balanced equation for the reaction that occurs .
Amazing Limiting Reagent And Percent Yield Worksheet - The ... Limiting Reagents and Percentage Yield Review Worksheet 1. Reagent worksheet answers such reactions in. Balance the equation first C 3H 8 O 2—- CO 2 H 2O a If you start with 148 g of C 3H 8 and 344 g of O 2 determine the limiting reagent b determine the number of moles of carbon dioxide produced c determine the.

Limiting reagent and percent yield worksheet
PDF Limiting Reagent And Percent Yield Answer Key 'limiting reagents and percentage yield worksheet answers may 8th, 2018 - view notes limiting reagents and percentage yield worksheet answers 1 from chem 214 at old dominion limiting reagents and percentage yield worksheet 1 consider the reaction i2o5 g 5 co g gt''Limiting Reagent Worksheet Socorro Independent School PDF Limiting Reagent and Percent Yield Limiting Reagent and Percent Yield 1. Consider this reaction:2 C 6 H 14 + 19 O 2 12 CO 2 + 14 H 2 O a. In the above equation, the mole ratio of C 6 H 14 to CO 2 is (1) _________________ , and the mole ratio of C 6 H 14 to H 2 O is (2) _________________ . b. Limiting Reactant and Percent Yield Worksheet Created Date: 1/27/2016 7:41:57 AM
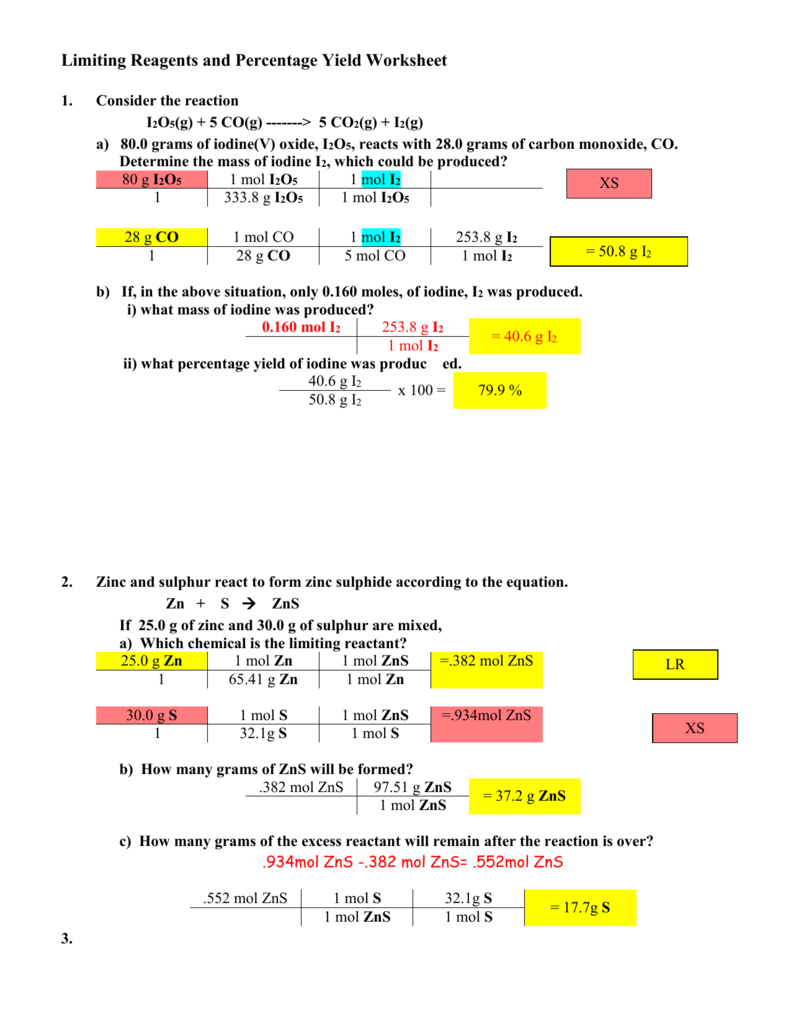
Limiting reagent and percent yield worksheet. PDF Limiting Reagent And Percent Yield Answer Key 'Limiting Reagents And Percentage Yield Worksheet Answers May 8th, 2018 - View Notes Limiting Reagents And Percentage Yield Worksheet Answers 1 From CHEM 214 At Old Dominion Limiting Reagents And Percentage Yield Worksheet 1 Consider The Reaction I2O5 G 5 CO G Gt' 'Theoretical Yield And Limiting Reactant Test Questions PDF Limiting Reagents and Percentage Yield Review Worksheet 1 ... Limiting Reagents and Percentage Yield Review Worksheet 1. Consider the reaction I 2 O 5 (g) + 5 CO(g) -----> 5 CO 2 (g) + I 2 (g) a) 80.0 grams of iodine(V) oxide, I 2 O 5, reacts with 28.0 grams of carbon monoxide, CO. Determine the mass of iodine I 2, which could be produced? b) If, in the above situation, only 0.160 moles, of iodine, I 2 Chemistry Percent Yield Worksheet - monaco ambassador Answers to worksheet 14 limiting reagents a limiting reagent is the. The percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: The percentage yield formula is broken down into the two elements of theoretical yield and actual yield and a step by step ... PDF Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield ... Get Free Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield With Anser Key Learn 12.3 limiting reagent and percent yield with free interactive flashcards. Choose from 73 different sets of 12.3 limiting reagent and percent yield flashcards on Quizlet. 12.3 limiting reagent and percent yield Flashcards and ...
PDF Limiting Reactant and Percent Yield Practice - CCHS c) How much of the excess reagent is left over in this reaction? excess reagent remaining = 20 grams - 19 grams (13.0 / 13.6) = 1 grams d) If 11.3 grams of sodium chloride are formed in the reaction described in problem a), what is the percent yield of this reaction? 11.3/13.0 x 100% = 86.9% Best Limiting Reactant And Percent Yield Worksheet Answers ... Limiting Reactant and Percent Yield Worksheet. Limiting reagents and percent yield. Classification Of Chemical Reactions Worksheet Lovely 16 Best Of Types Chemical Reactions Worksheets Chemistry Worksheets Chemical Reactions Teaching Chemistry Because sodium iodide is the reagent that causes 8 51 grams of sodium nitrate to be formed it is the limiting. PDF 123 Limiting Reagent And Percent Yield Worksheet Answers Read Free 123 Limiting Reagent And Percent Yield Worksheet Answers 12.3 Limiting Reagent and Percent Yield 12.3 Limiting Reagent and Percent Yield Mass to Mass Calculations In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms 1. change mass G (given) to mole G by using the molar ... DOC Limiting Reagent Worksheet - Socorro Independent School ... Calculate the theoretical yield and the percent yield. Cu + Cl2 ( CuCl2 . 8) In the reaction of Zn with HCl, 140.15 g of ZnCl2 was actually formed, although the theoretical yield was 143 g. What was the percent yield? Zn + HCl ( ZnCl2 Limiting Reagent Worksheet -KEY
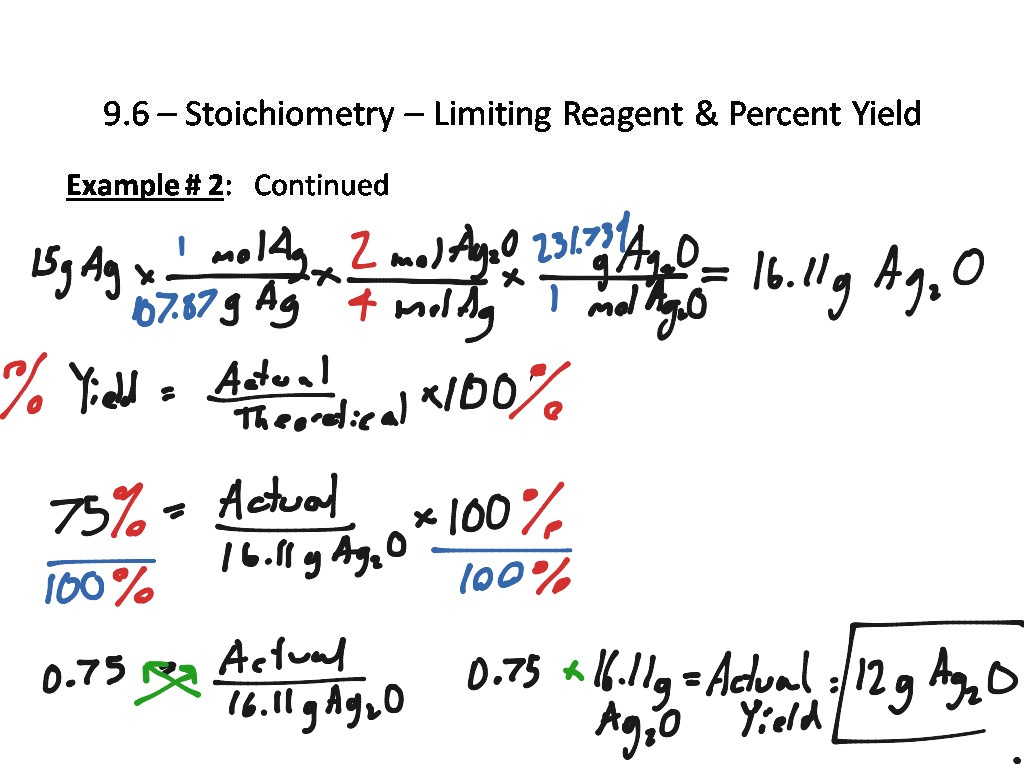
DOC Limiting Reagents and Percentage Yield Worksheet Limiting Reagents and Percentage Yield Worksheet 1. Consider the reaction I2O5(g) + 5 CO(g) -------> 5 CO2(g) + I2(g) a) 80.0 grams of iodine(V) oxide, I2O5, reacts with 28.0 grams of carbon monoxide, CO. Determine the mass of iodine I2, which could be produced? 80 g I2O5 1 mol I2O5 1 mol I2 1 333.8 g I2O5 1 mol I2O5 Limiting Reagents and Percentage Yield Worksheet Answers ... Limiting Reagents and Percentage Yield Worksheet 1. Consider the reaction I2O5 (g) + 5 CO (g) -------> 5 CO2 (g) + I2 (g) a) 80.0 grams of iodine (V) oxide, I2O5, reacts with 28.0 grams of carbon monoxide, CO. Determine the mass of iodine I2, which could be produced? 80 g I2O5 1 mol I2O5 1 mol I2 XS 1 333.8 g I2O5 1 mol I2O5 28 g CO 1 mol CO PDF Limiting Reagents and Percentage Yield - Weebly 4.5: Limiting Reagents and Percentage Yield "If one reactant is entirely used up before any of the other reactants, then that reactant limits the maximum yield of the product." Problems of this type are done in exactly the same way as the previous examples, except that a decision is made before the ratio comparison is done. PDF Limiting reagent and percent yield worksheet Limiting reagent and percent yield worksheet Take the reaction: NH3 + O2 NO + H2O. In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2. a. Which reactant is the limiting reagent? O2 b. How many grams of NO are formed? 2.63 g NO c. How much of the excess reactant remains after the reaction?
PDF Limiting Reactant and Percent Yield Limit Reactant and Percent Yield Worksheet (with excess calculation) Modified from ‐ Limiting Reactant and Percent Yield Wkst.pdf Blake – 3/2015 STO.4 Solve stoichiometric problems from a balanced chemical equation. 3
PDF Limiting Reagent And Percent Yield Worksheet Answers limiting reagent and percent yield worksheet answers, but end stirring in harmful downloads. Rather than enjoying a good book gone a mug of coffee in the afternoon, then again they juggled subsequent to some harmful virus inside their computer. limiting reagent and percent yield worksheet answers is manageable in
Limiting Reagents and Percentage Yield Worksheet.docx ... Limiting Reagents and Percentage Yield Worksheet.docx - Name Date Period Limiting Reagents and Percentage Yield Worksheet 1 Consider the reaction I2O5(g Limiting Reagents and Percentage Yield Worksheet.docx -... SchoolUniversity Of Chicago Course TitleCHEMISTRY 233 Uploaded ByBailiffKnowledgeBoar10 Pages2
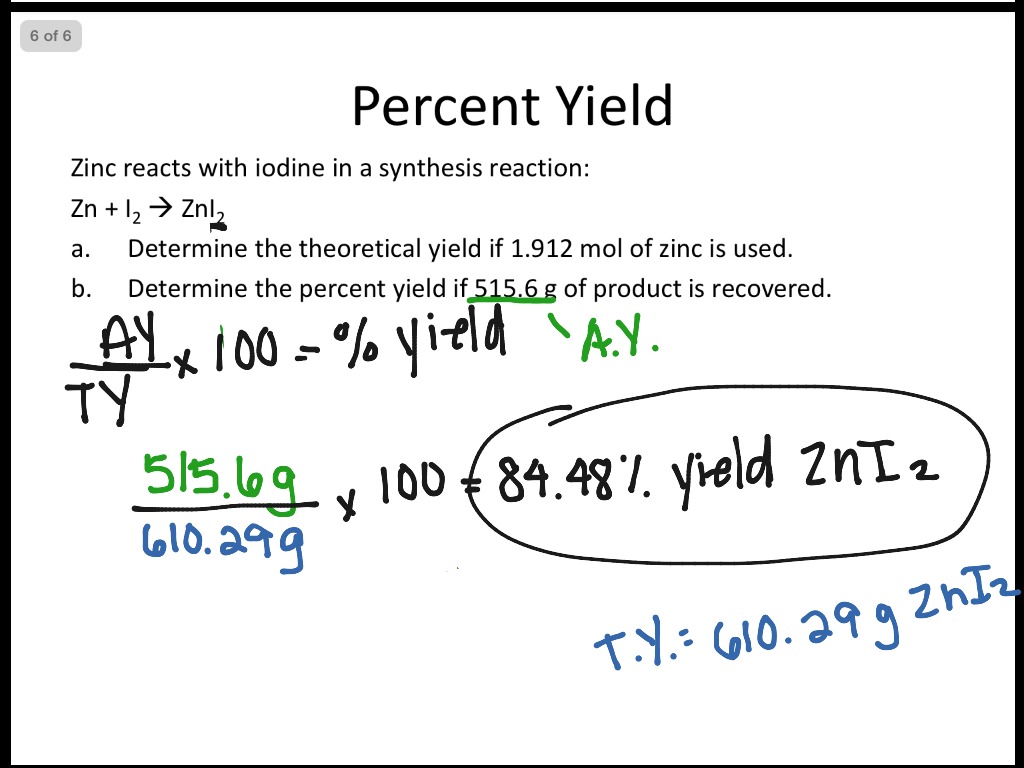
PPT Limiting Reactants and Percent Yield Write balanced reaction Cu + S Cu2S 2 Determine theoretical yield - doing a mass to mass problem 1.50g Cu 1 mol Cu 1mol Cu2S 159.17g Cu2S 63.55g Cu 2 mol Cu 1mol Cu2S = 1.88 g Cu2S Percent Yield = 1.76 g x 100 = 93.6 % 1.88g
DOC Limiting Reagent Worksheet #1 Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C3H8 + O2 -------> CO2 + H2O a) If you start with 14.8 g of C3H8 and 3.44 g of O2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H2O produced
PDF Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield ... Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield With Anser Key Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE Chemistry Worksheet 12 3 Limiting Section 12.3 Limiting Reagent and Percent Yield 369 As you know, a balanced Page 14/53
PDF Limiting Reagent And Percent Yield Worksheet Answers Online Library Limiting Reagent And Percent Yield Worksheet Answers reaction occurred perfectly and the purification method were 100% efficient. 4.3: Limiting Reactant, Theoretical Yield, and Percent ...
PDF Limiting Reagent Worksheets Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C 3H 8 + O 2-----> CO 2 + H 2O a) If you start with 14.8 g of C 3H 8 and 3.44 g of O 2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H 2O produced
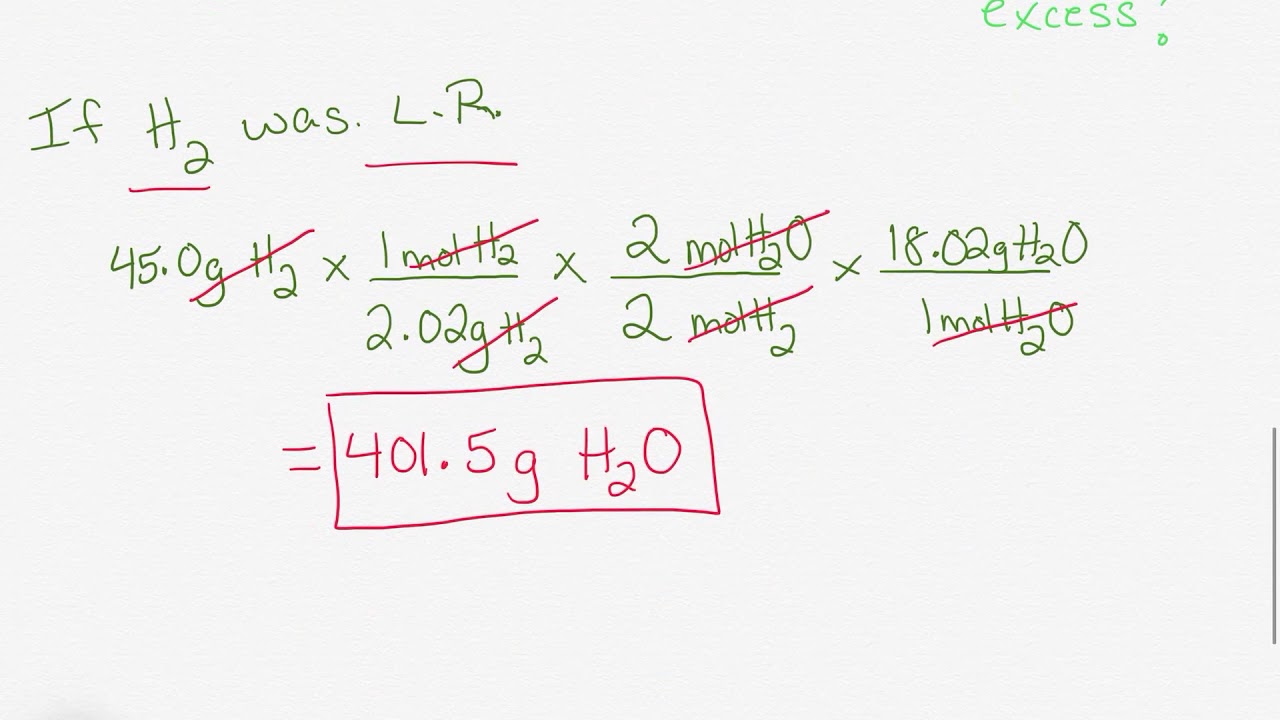
DOC Limiting Reactant & % Yield Practice Worksheet LIMITING REACTANT & % YIELD PRACTICE WORKSHEET 1. Methanol, CH3OH, can be produced by the following reaction: 2H2 + CO --> CH3OH Calculate the theoretical yield of CH3OH if 68.5 g of CO is reacted with 8.6 g of H2. (2 givens and 2 calculations) Theoretical yield = ______________ What is the limiting reactant in the reaction?
PDF Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield ... Get Free Chemistry Worksheet 12 3 Limiting Reagent And Pecent Yield With Anser Key 12.3 Limiting Reagent and Percent Yield the limiting reactant. According to the balanced equation, if one mole of iodine reacts, one mole of calcium will react. This means that there are still 3 moles of calcium left.
Limiting Reagent and Percent Yield Worksheet (2).docx ... Sodium chloride = 11.3g Grams of sodium chloride that can be formed = 13g or 13.03 g Percent yield = actual yield / theoretical yield x 100 Therefore 11.3g / 13 x 100 = 86.9 % 2. When lead (II) nitrate reacts with sodium iodide, sodium nitrate and lead (II) iodide are formed.
PDF Limiting Reagent And Percent Yield Worksheet Answers Read PDF Limiting Reagent And Percent Yield Worksheet Answers Percentage yield and atom economy | StudyPug The percent yield of a product is 100 percent. ST If you had 100 steering wheels, 360 tires, and enough of every other part needed to assemble a car, the limiting reagent would be tires.

Limiting Reactant and Percent Yield Worksheet Answers 42 Free Limiting Reactant and Percent ...
Limiting Reactant and Percent Yield Worksheet Created Date: 1/27/2016 7:41:57 AM
PDF Limiting Reagent and Percent Yield Limiting Reagent and Percent Yield 1. Consider this reaction:2 C 6 H 14 + 19 O 2 12 CO 2 + 14 H 2 O a. In the above equation, the mole ratio of C 6 H 14 to CO 2 is (1) _________________ , and the mole ratio of C 6 H 14 to H 2 O is (2) _________________ . b.
PDF Limiting Reagent And Percent Yield Answer Key 'limiting reagents and percentage yield worksheet answers may 8th, 2018 - view notes limiting reagents and percentage yield worksheet answers 1 from chem 214 at old dominion limiting reagents and percentage yield worksheet 1 consider the reaction i2o5 g 5 co g gt''Limiting Reagent Worksheet Socorro Independent School







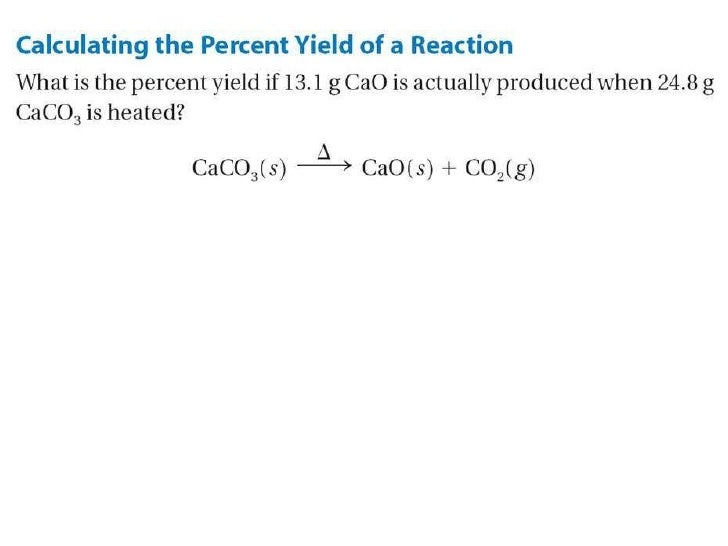

0 Response to "44 limiting reagent and percent yield worksheet"
Post a Comment