39 percent composition and empirical formula worksheet
PDF 7-21 % Composition and Empirical Formulas wkst Part 2: Empirical Formulas Work each of the following problems. SHOW ALL WORK. 1. A compound is found to contain 63.52 % iron and 36.48 % sulfur. Find its empirical formula. 2. In the laboratory, a sample is found to contain 1.05 grams of nickel and 0.29 grams oxygen. Determine the empirical formula. PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? 3. What's the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon, and 65.0% oxygen? 4.
PDF 11. Empirical Formula Worksheet II EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.

Percent composition and empirical formula worksheet
% Composition, Empirical And Molecular Formula Teaching ... Percent Composition, Empirical & Molecular Formulas Task Cards by Chemistry Corner 7 $3.50 Zip Differentiate this resource to meet your needs with the included editable task cards! You will find these task cards to be a very versatile teaching tool. PDF Part A: Mass Percent Composition - csus.edu Part A: Mass Percent Composition An element's mass percent composition is constant for each compound no matter how much of the ... CHM 4, PAL - Mass percent and empirical formulas Student name: 4 6) Diethylene glycol (used in antifreeze blending) has the composition: 45.27% C, 9.50 % H, and 45.23% O by mass. Its molar mass is 106.12 g/mol. Empirical Formula Worksheets - DSoftSchools Empirical Formula Worksheets. admin October 23, 2020. Some of the worksheets below are Empirical Formula Worksheets, several exam style questions like determine the empirical formula of a compound that contains 53.70% iron and 46.30% sulfur, … solutions are provided at the end of each worksheet. Basic Instructions.
Percent composition and empirical formula worksheet. PDF Percent Composition, Empirical and Molecular Formulas Empirical Formula Determination 1.If given percentages of elements, assume you have 100 grams of the compound. Determine moles of each element in 100 grams of the compound. 2.Divide each value of moles by the smallest of the mole values. 3.Multiply each number by an integer to obtain all whole numbers. Worksheets - Empirical Formula - Arkansas State University 2. Determine the empirical formula from the percent composition for each of the following: a) 92.24 % C; 7.76 % H. b) 36.48 % Na; 25.44 % S; 38.08 % O DOC Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet Solutions. 1) What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? C3H3O. 2) If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? C6H6O2 _____ Empirical Formulas And Percent Composition Worksheets & Teaching ... Percent Composition, Empirical Formulas, & Molecular Formulas Worksheet by Chem Queen 3 $1.50 Zip Here's a great practice worksheet that includes 10 questions.
PDF 7-21a % Composition and Empirical Formulas wkst-Key Worksheet: % Composition and Name _____ Empirical Formulas CHEMISTRY: A Study of Matter © 2004, GPB 7.21a KEY Part 1: % Composition Calculate the percent composition of the following compounds. SHOW ALL WORK. HCl 1.0 g + 35.5 g = 36.5 g H (1.0 g/36.5 g) x 100 % = 2.7 % H Cl (35.5 g/36.5 g) x 100 % = 97.3 % Cl K 2CO 3 DOCX Percentage Composition and Empirical & Molecular Formula Chemistry: Percentage Composition and Empirical & Molecular Formula Solve the following problems. Show your work, and always include units where needed. 1. A compound is found to contain 36.5% Na, 25.4% S, and 38.1% O. Find its empirical formula. 2. Find the empirical formula of a compound that is 53.7% iron and 46.3% sulfur. 3. PDF Worksheet # C43: Percent Composition, Empirical Formulas, and Molecular ... Worksheet # C43: Percent Composition, Empirical Formulas, and Molecular Formulas 1. What is the percent composition of SO 2 S = _______________ % O = _______________ % 2. What is the percent composition of calcium phosphate? Ca = ______________ % P = ______________ % 0 = ______________ % 3. Mole 7 Worksheet : Percent Composition And Molecular Formula Worksheet Percent composition and molecular formula worksheet. Show your work, and always include units where needed. H · 2.5 · =25% ; Determine the molecular formula for each compound described. Determine the molecular formula for each compound described. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen?
PDF Percent Composition and Molecular Formula Worksheet Write the molecular formulas of the following compounds: 5. A compound with an empirical formula of C 2 OH 4 and a molar mass of 88 grams per mole. 6. A compound with an empirical formula of C 4 H 4 O and a molar mass of 136 grams per mole. 7. A compound with an empirical formula of CFBrO and a molar mass of 254.7 grams per mole. 8. A compound ... PDF formula work sheet - Mister Chemistry Worksheet 7-3 Name Percent Composition & Empirical Formulas Period Glencoe Chemistry pp. 328-337 Show yeyr work to receive credit. Circle pour final answer. A. Calculate the percent composition for the following compounds cro 2. B. Calculate the percent by mass of iron in each ofthe following compounds. 3. iron (Ill) oxide 4. iron (Il) oxide PDF Worksheet #8 Empirical Formulas H O N O 4I Worksheet #8 Empirical Formulas 1. State the empirical formula for each of the following compounds: a) C 4H8; b) C 2H6O2; c) N2O5; d) Ba 3(PO 4)2; e) Te 4I16 2. What is the empirical formula for a compound that contains 0.063 mol chlorine and 0.22 mol oxygen? 3. What is the empirical formula for a compound that contains 26.1% carbon, 4.3% hydrogen DOC Percent Composition and Molecular Formula Worksheet Find the molecular formula for a compound with an empirical formula of CFBrO and a molar mass of 254.7 grams per mole.: A 50.51 g sample of a compound made from phosphorus and chlorine is decomposed. Analysis of the products showed that 11.39 g of phosphorus atoms were produced. Determine the mass of chlorine in the sample. Determine the percent composition of each element in the compound
Quiz & Worksheet - How to Calculate Percent Composition and Determine ... Worksheet 1. Which of the following calculations is NOT part of determining the empirical formula? Percentage composition Mass composition Composition in moles Moles to mass 2. What is the percent...
DOC PERCENTAGE COMPOSITION WORKSHEET - The Mole Find the empirical formula for a compound which contains 32.8% chromium and 67.2% chlorine. (CrCl3) NAME the compound which contains 0.463 g Tl (#81), 0.0544 g of carbon, 0.00685 g of hydrogen and 0.0725 g of oxygen by finding its empirical formula. (TlC2H3O2) What is the empirical formula for a compound which contains 67.1% zinc and the rest ...
DOC % Composition, Empirical Formula and Molecular Formula Worksheet - Weebly % Composition, Empirical Formula and Molecular Formula Worksheet A sample of an unknown compound with a mass of 0.847 g has the following composition: 50.51 % fluorine and 49.49 % iron. When this compound is decomposed into its elements, what mass of each element would be recovered? F = 0.4278g Fe = 0.4192g

Percent Composition and Molecular Formula Worksheet - Percent Composition and Molecular Formula ...
Empirical Formula Practice Test Questions - ThoughtCo Updated on July 03, 2019. The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound. This 10-question practice test deals with finding empirical formulas of chemical compounds. A periodic table will be required to complete this practice test.
Percent Composition Empirical Formula Worksheet - Isacork Percent Composition Empirical Formula Worksheet. Answers to worksheet #8 empirical formulas to calculate empirical formulas, follow the steps outlined below: S = _____ % o = _____ % 2. Percent Composition Empirical Formulas Molecular Formulas from fdocuments.in A compound with an empirical formula of c4h4o and a molar mass of 136 grams per mole.
PDF Georgetown Independent School District / GISD Home numbers of atoms in the compound. Multiple compounds can have the same empirical formula. Glucose, Cd-11206, contains carbon, hydrogen, and oxygen. The ratio of carbon to hydrogen to oxygen is CH20 is the empirical formula for glucose. Since the percent composition of each element in an empirical formula represents the ratio in which they
PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? 3. What's the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon, and 65.0% oxygen? 4.
PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1) What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2) If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? _____ 3) What's the empirical formula of a molecule containing 18.7% lithium,


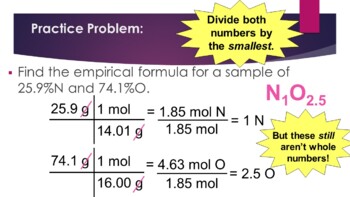
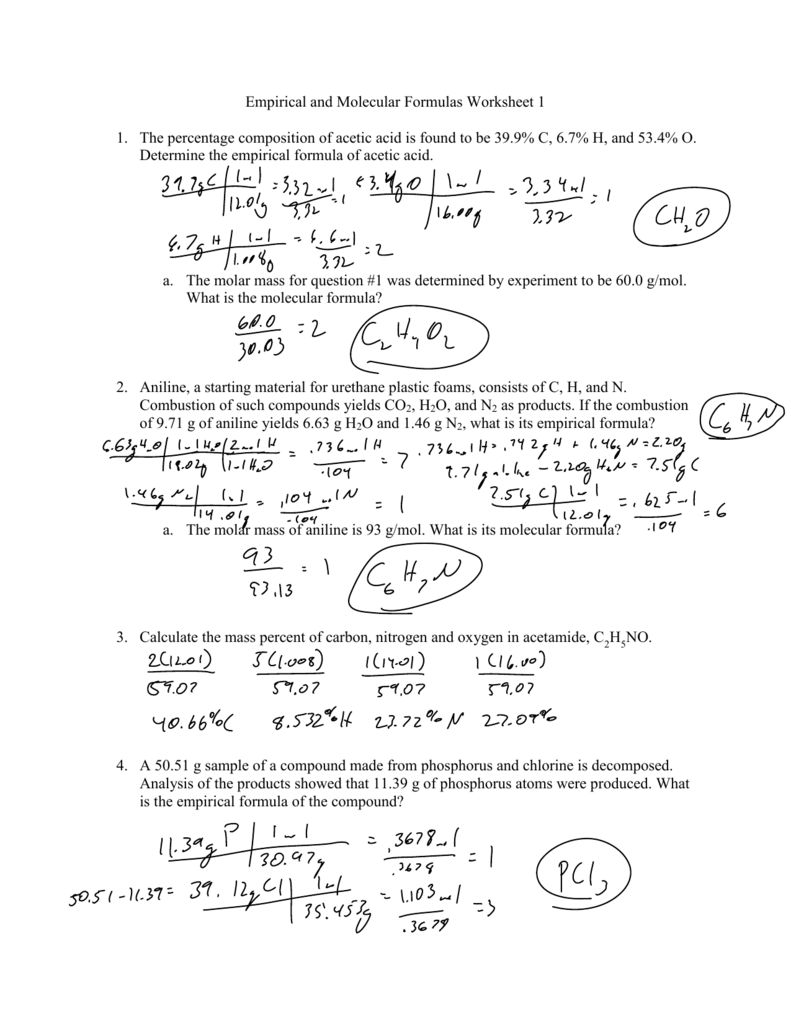

0 Response to "39 percent composition and empirical formula worksheet"
Post a Comment