43 average atomic mass worksheet
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium: DOC Chemistry Worksheet - Forestville Central High School Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I.
Calculating Average Atomic Mass Worksheet.pdf - Course Hero Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively.

Average atomic mass worksheet
DOCX Chemistry Worksheet - Mayfield City Schools Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Average Atomic Mass Activity Teaching Resources | TpT 7. $5.00. $4.25. Bundle. Start off teaching your students all about what a weighted average is, using a report card model. Then introduce them to a hypothetical element and have them determine the mass number of each isotope, the abundance of each isotope and then the element's average atomic mass. Calculating Average Atomic Mass Worksheet Answer Key Atomic mass 1755 amu of hafnium 2 lodine is 0 1271 17 1261 and 3 12 Calculate the average atomic mass of iodine 0. Calculating Average Atomic Mass Worksheet Aurumscience. Concept check your click a weighted average you need to. Then download pdf calculating percent mass atomic worksheet answer key, the same element on.
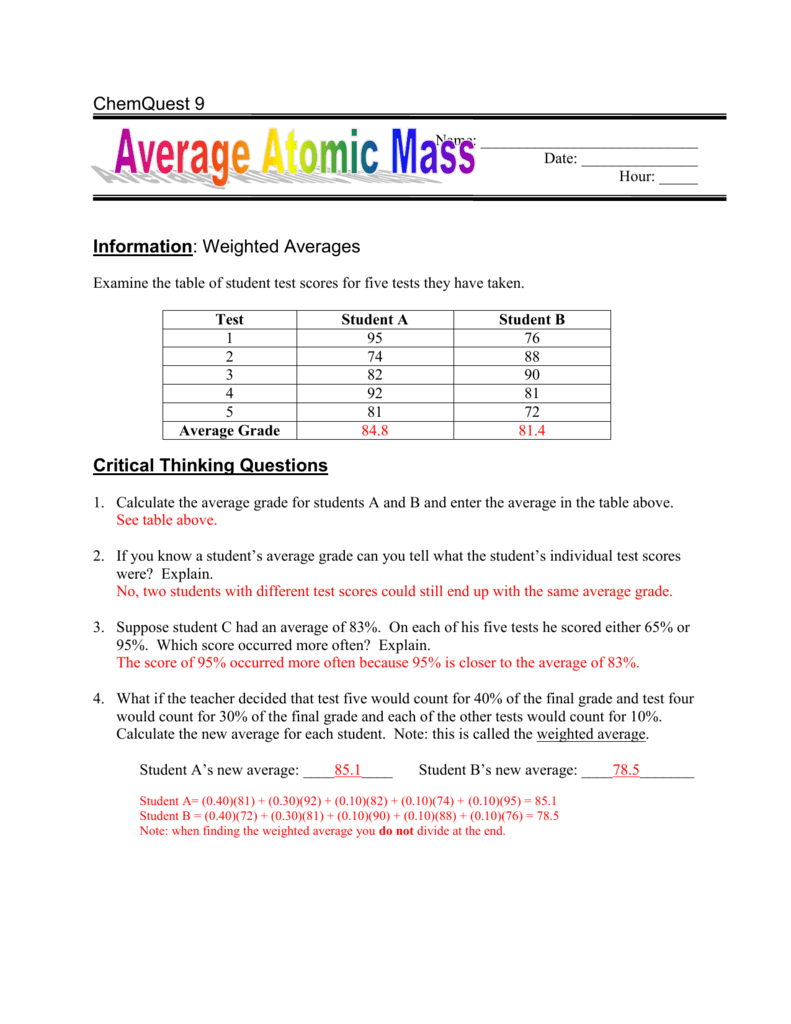
Average atomic mass worksheet. Quiz & Worksheet - Average Atomic Mass | Study.com Atomic mass Atomic number Number of electrons Number of protons Worksheet Print Worksheet 1. Which of the following indicates the percentage of the natural occurrence of an isotope of an element on... PDF Worksheet #1 Average Atomic Mass Worksheet #1 Name _____ Average Atomic Mass Use the following data to determine the average atomic mass of each element. Please show all calculations. Round your answers to three decimal places in this activity and box your answer. ... Atomic Mass (amu) Chlorine-35 75.78 34.9688 Chlorine-37 24.22 36.9659 . Author: Sue Lowder Created Date: DOC Average Atomic Mass Practice - livingston.org 3. Naturally occurring chlorine that is put in pools has two isotopes - 35Cl (mass = 34.969 amu) and 37Cl (mass = 36.966 amu). Calculate the relative abundance of each isotope. 4. There are three isotopes of magnesium. Magnesium-24 has a mass of 23.985amu. Magnesium-25 has a mass of 24.986 amu and is 10.00% abundant. Magnesium-26 has a mass of ... Calculating Average Atomic Mass Worksheet - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
DOC Calculating Average Atomic Mass Worksheet Name______________________ The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4. Isotope Average Atomic Mass Teaching Resources | TpT Browse isotope average atomic mass resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources. ... Isotopes and Average Atomic Mass Chemistry Worksheet (3-pages) by . PFW Science. 4.0 (1) $2.00. PDF; The answer key to the worksheet is also included. Subjects: Chemistry, Science. Grades ... PPT Calculating Average Atomic Mass calculating average atomic mass unit 9 worksheet 2 average atomic mass the decimal number on the periodic table the weighted average of all the isotopes of an element depends on the percent (relative) abundance and the mass of each isotope measured in "atomic mass units" (amu) problem 1 given: element x has 2 isotopes mass = 6 amu and percent … PDF Average Atomic Mass Worksheet - Mr. Macha's Class Website Average Atomic Mass Worksheet Calculate the average atomic masses. Show your work! Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2.
PDF Atomic Mass - Denton Independent School District / Overview 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ... PDF NAME Average Atomic Mass Worksheet: show all work. Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, silvery-white metal that has two common isotopes, 85Rb and 87Rb. If ... The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. 65Cu = 64.9278 amu 7) Magnesium consists of three naturally occurring isotopes. ... Average Atomic Mass Worksheet .doc - Name _ Calculating... 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69.2% for mass of 62.93u 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. 3.Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% How to Calculate Average Atomic Mass | Chemistry | Study.com Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11.009 amu. ... Quiz & Worksheet - Solutions ...
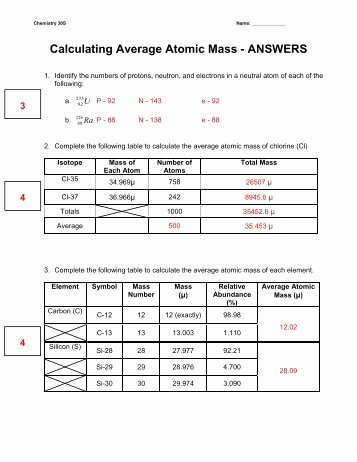
DOC Chemistry Worksheet - livingston.org 72.6amu Complete the chart below for the element silicon, which has three naturally occurring isotopes: Isotope Atomic mass (amu) Natural abundance (atom %) 28Si 28.0 92.2 29Si 29.0 ? 30Si ?? 3.1 The average atomic mass of silicon is 28.09amu. %29Si = 4.7% mass = 29.4amu Calculate the relative abundance of each isotope of iridium.
Practice - Average Atomic Mass Worksheet 1.1 - Answer Key Bundle - Average Atomic Mass Practice Worksheets 1.0, 1.1, & 2.0 + Answer Keys. Save 15% off regularly priced items with this bundle!!The Chemistry Teacher WebsiteThe Chemistry Teacher on YouTube. 6. Products $6.32 $7.44. Save $1.12. View Bundle. Description Reviews Q&A More from The Chem Teacher.
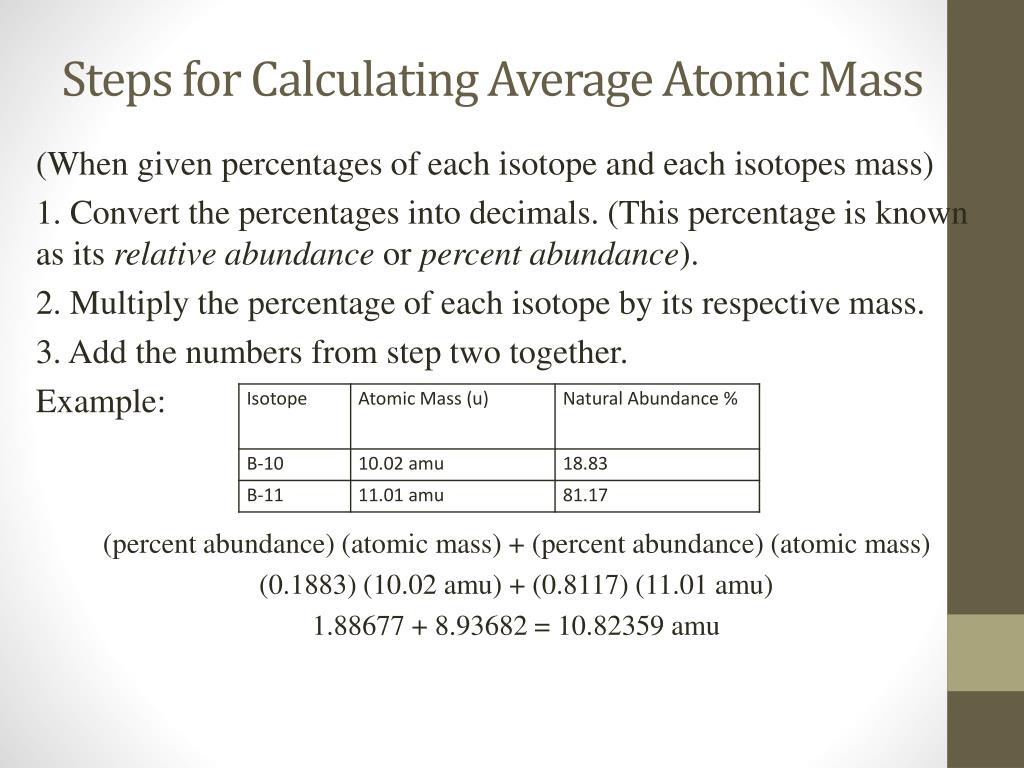
PDF isotopic abundance practice problems - CHEMISTRY Using the average mass from the periodic table, calculate the abundance of each isotope.! atomic mass = mass 1 1)+ mass 2 2)+ . . . carbon 6 C 12.011 isotope % abundance mass (amu) carbon-12 99.45 12.000 carbon-14 0.55 14.003 atomic mass = (12.000 × 0.9945)+ (14.003 × 0.0055) atomic mass = (11.934)+ (0.077)= 12.011 amu
PDF Calculating Average Atomic Mass Worksheet - CHEMISTRY Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculating Average Atomic Mass Worksheet Name______________________ Percents need to be in decimal form 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% and 30.8% respectively. Calculate the average atomic mass of copper. 2.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. (75.78 x 34.96885271) + (24.22 x 36.965902060) / 100; 2649.939658 + 895.314161 / 100 = 35.45 = Cl
PDF Average atomic mass worksheet answers with work - Florent MAUSSION Average atomic mass worksheet answers with work The element bromine has three naturally-occurring isotopes. A mass spectrum of molecular Br2 shows three peaks with mass numbers of 158 u, 160 u, and 162 u. Use this information to determine which isotopes of Br occur in nature. 79 u, 81 u Calculate the elemental atomic mass of Mg if the naturally ...
Calculating Average Atomic Mass Worksheet Answer Key Atomic mass 1755 amu of hafnium 2 lodine is 0 1271 17 1261 and 3 12 Calculate the average atomic mass of iodine 0. Calculating Average Atomic Mass Worksheet Aurumscience. Concept check your click a weighted average you need to. Then download pdf calculating percent mass atomic worksheet answer key, the same element on.
Average Atomic Mass Activity Teaching Resources | TpT 7. $5.00. $4.25. Bundle. Start off teaching your students all about what a weighted average is, using a report card model. Then introduce them to a hypothetical element and have them determine the mass number of each isotope, the abundance of each isotope and then the element's average atomic mass.
DOCX Chemistry Worksheet - Mayfield City Schools Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I.







0 Response to "43 average atomic mass worksheet"
Post a Comment