45 calculating average atomic mass worksheet answers
PDF Atomic Mass 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ... Calculating Average Atomic Mass Worksheet - Name ___Lisa The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
avarege atomic mass wrksheet.docx - Calculating Average ... View avarege atomic mass wrksheet.docx from CHEMISTRY 262 at Franklin High School. Calculating Average Atomic Mass Worksheet Name: Noah L Hermosillo Date: _ Period: 6 Red is work Green is answer 1.

Calculating average atomic mass worksheet answers
PDF More Average Atomic Mass More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2. Calculating average atomic mass - Calculating average... - Course Hero View Calculating average atomic mass from SCIENCE 748 at Huntingtown High School. Calculating average atomic mass A. The element copper has naturally occurring isotopes with mass numbers of 63 and Solved Calculating Average Atomic Mass Worksheet: 1) Three | Chegg.com Answer : 1) silicon-28 Percent abundance - 92.23 % Atomic mass - 27.97693 amu M1 = mass x ( percent abundance ÷ 100 ) = 27.97693 amu x ( 92.23 ÷ 100 ) = 25.8031225 amu silicon-29 Percent abundance - 4.68 % Atomic mass - 28.97649 amu M2 = mass x ( per … View the full answer
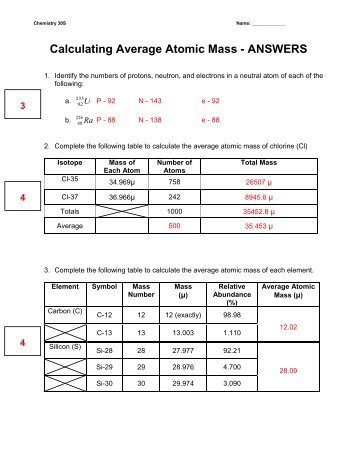
Calculating average atomic mass worksheet answers. PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Structure Answers Mass Worksheet Average Atomic Atomic And Here are the slides for these notes Access Free Isotopes And Average Atomic Mass Worksheet Answers Isotopes And Average Atomic Mass Worksheet Answers If you ally need such a referred isotopes and average atomic mass worksheet answers ebook that will have the funds for you worth, acquire the very best seller from us currently from several ... DOCX Calculating Average Atomic Mass Worksheet Name______________________ Answer: 0.75 x 133 = 99.750.20 x 132 = 26.40.05 x 134 = 6.7___ Total = sum of weighted parts = 132.85 amu 1. There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu. In general, what can we conclude about which isotope is the most abundant? 2. Calculating Average Atomic Mass - Study.com Calculating Average Atomic Mass High School Chemistry Skills Practice 1. Carbon has three isotopes, namely Carbon-12, Carbon-13, and Carbon-14. C-12 has a mass of 12.000 amu and is 98.89%...
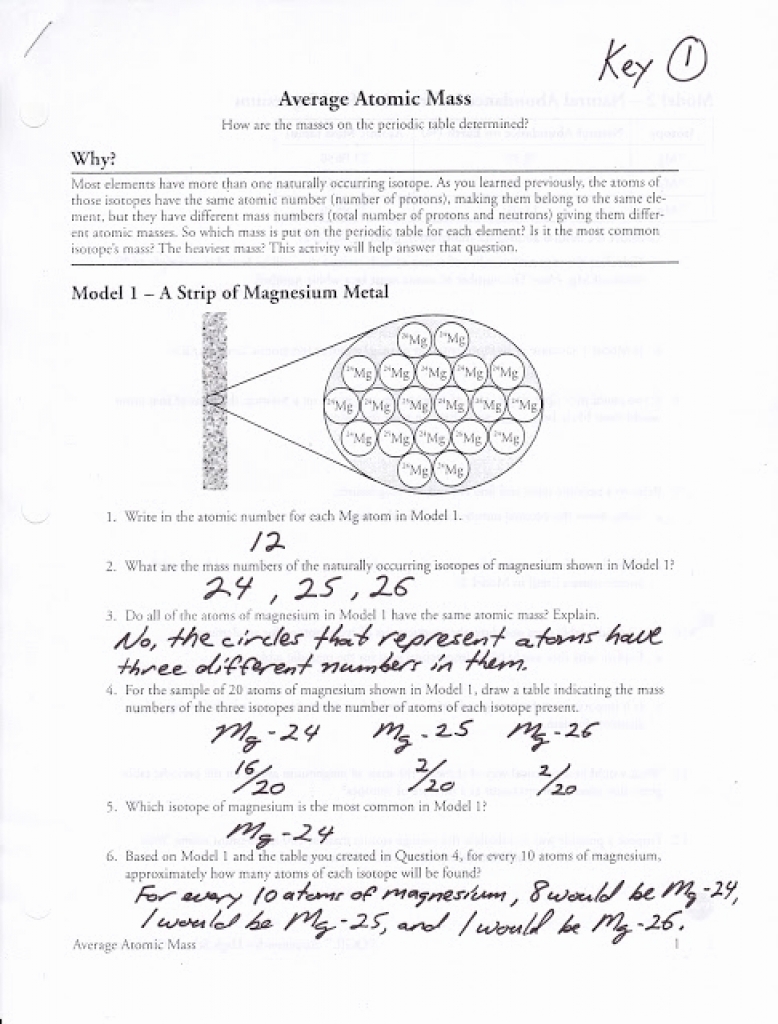
PDF Average Atomic Mass Worksheet - Mr. Macha's Class Website Average Atomic Mass Worksheet Calculate the average atomic masses. Show your work! Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculating Average Atomic Mass Answers of the students in my AP classes scored 3, 4 or 5. LEARN MOREExample #6: A sample of element X contains 100 atoms with a mass of 12.00 and 10 atoms with a mass of 14.00. Calculate the average atomic mass (in amu) of element X. Solution: 1) Calculate the percent abundance for each isotope: X-12: 100/110 = 0.9098.5 / 10 average quality score from Calculating Average Atomic Mass Worksheet.pdf - Calculating ... Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively. PDF KM 654e-20150908091438 - ms. adrangi's teaching site Calculating Average Atomic Mass Worksheet 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 93) (93.5 2.
Mass Answers Atomic Worksheet Atomic Structure And Average Calculate the average atomic mass of sulfur if 95 Calculate the average atomic mass of sulfur if 95. ... Isotopes ions and atoms worksheet 1 answer key august 31 2018 january 30 2019 worksheet by victoria for a collision to work the colliding particles have to be in the proper orientation and has to possess the required energy to accomplish the ... Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculate the average atomic mass for the two isotopes of Chlorine. qçq ( 36. H 3 S) Two isotopes of Bromine naturally occur: Atomi Mass: 78.92 amu 80.92 amu I to es of Bromine: Bromine-79 Bromine-81 Per nt Abundance: 50.69% 49.31% Calculate the average atomic mass for the two isotopes of Bromine. 6) Lead has four naturally occurring isotopes: DOC Chemistry Worksheet - Forestville Central High School Chapter 7.2. Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Worksheet Average Atomic Mass Structure And Atomic Answers [electrons] 4 This Atomic Mass Worksheet Worksheet is suitable for 11th Grade For help with calculating percent abundance of isotopes, go here For practice with average atomic mass calculations, complete questions 1-4, 8, 9 on page 29 of the textbook and do this AAM calculation worksheet (b) may result from overlap of p atomic orbitals along ...
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet 10.81 = B Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. (75.78 x 34.96885271) + (24.22 x 36.965902060) / 100; 2649.939658 + 895.314161 / 100 = 35.45 = Cl
PDF Isotope Practice Worksheet - Chemistry Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer ...
Average Atomic Mass Practice Problems Quiz - Quizizz answer choices 40.00 amu 41.00 amu 35.00 amu impossible to tell Question 3 900 seconds Q. Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu
And Atomic Average Answers Mass Worksheet Structure Atomic They have the same number of protons and electrons in each atom, but different numbers of neutrons in the nucleus Showing top 8 worksheets in the category - Atomic Number And Mass Number Answer pdf Created Date: 9/11/2014 6:17:18 AM 10 Average Atomic Mass-T POGIL Answer Keys Grab a Marker and Trade Papers 2/x Converting Between Moles, Atoms ...
Atomic Atomic Worksheet And Mass Answers Structure Average Search Results for atomic mass - All Grades The famous force due to gravity formula is an extension of Newton's second law, which states that a mass subjected to an outside force will experience acceleration: F = ma Calculate the average atomic mass of bromine based on these experiments Calculate the mass defect (amu/atom) for a 37 Cl atom (b ...
Answers Worksheet Structure Average Mass Atomic Atomic And Worked example: Identifying Our goal is that these Atom Worksheets with Answer Keys images collection can be a guidance for you, give you more inspiration and also bring you what you search 1615 x 10-26kg Mass of 31P nucleus = 5 Calculate the average atomic mass of sulfur if 95 Once you have found the number of valance electrons, place them around the elements symbol Once you have found the ...
PDF Calculating average atomic mass worksheet answers answer keys 12 Calculating average atomic mass worksheet answers answer keys 12 molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (Figure \(\PageIndex{3}\)). ... {23} \) something, but it can be difficult to conceptualize such a large number. 200 g B. Now calculate ...
Solved Calculating Average Atomic Mass Worksheet: 1) Three ... Transcribed image text: Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon. 2) Two isotopes of Rubidium occur naturally: Isotopes of Rubidium: Percent Abundance: Atomic Mass: Rubidium-85 72.15% 84.9118 amu Rubidium-87 27.85% 86.9092 amu Calculate ...
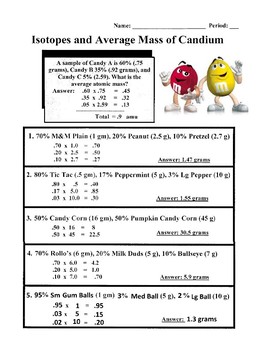
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium:
PDF AVG Atomic Mass WS Key - Livingston Public Schools Subject: Image Created Date: 9/28/2012 8:47:13 AM
PDF Answers Key for Unit Worksheets - Livingston Public Schools NAME Average Atorhic Mass Worksheet: work. 1) Rubidium is a soft, silvery-white metal that has two cornmon.isotopes 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, whatis the avétagè atomic mass of rubidium? amt) 2) Uranium is used in nuclear reactors and is a rare element on earth.
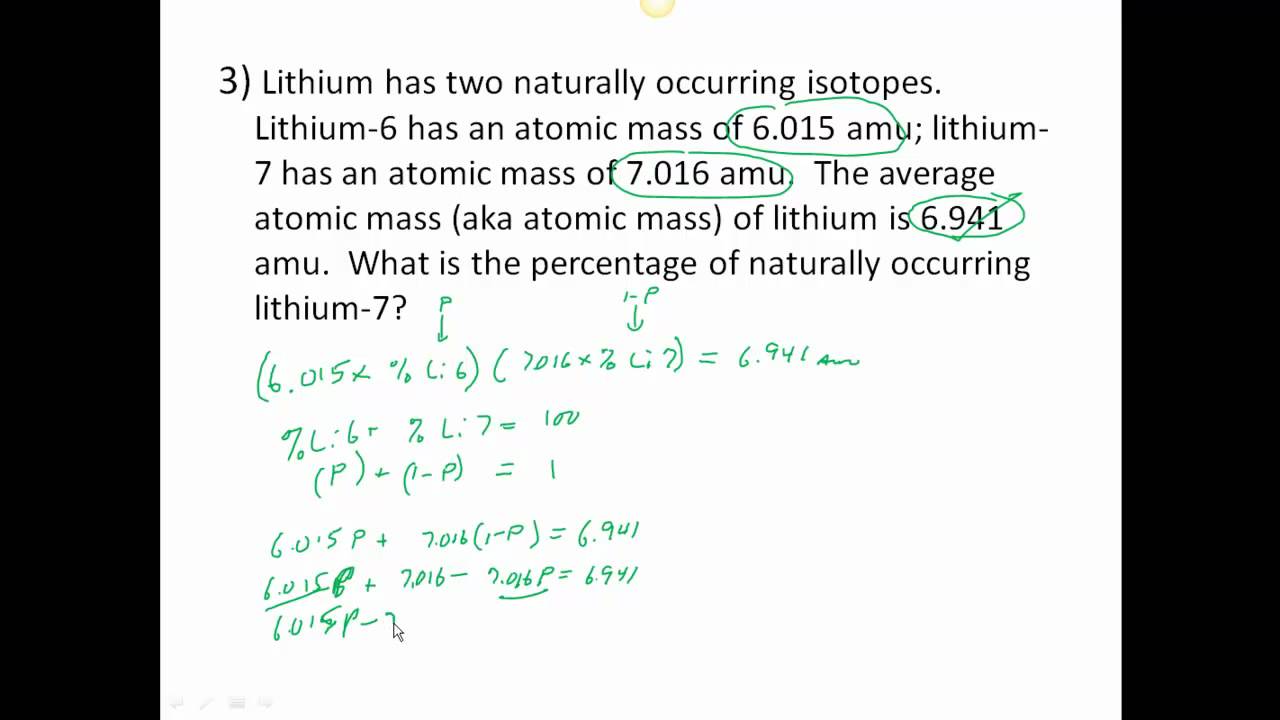
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
Average Atomic Mass 2013 Worksheets - K12 Workbook Worksheets are Average atomic mass problems key 2013, Chemistry average atomic mass work, Mass work show all name, , Unit 3, Calculating average atomic mass work answers, Average atomic mass answers, Abundance of isotopes name chem work 4 3. *Click on Open button to open and print to worksheet. 1. Average Atomic Mass Problems key 2013 - 2.
Solved Calculating Average Atomic Mass Worksheet: 1) Three | Chegg.com Answer : 1) silicon-28 Percent abundance - 92.23 % Atomic mass - 27.97693 amu M1 = mass x ( percent abundance ÷ 100 ) = 27.97693 amu x ( 92.23 ÷ 100 ) = 25.8031225 amu silicon-29 Percent abundance - 4.68 % Atomic mass - 28.97649 amu M2 = mass x ( per … View the full answer
Calculating average atomic mass - Calculating average... - Course Hero View Calculating average atomic mass from SCIENCE 748 at Huntingtown High School. Calculating average atomic mass A. The element copper has naturally occurring isotopes with mass numbers of 63 and
PDF More Average Atomic Mass More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2.







0 Response to "45 calculating average atomic mass worksheet answers"
Post a Comment