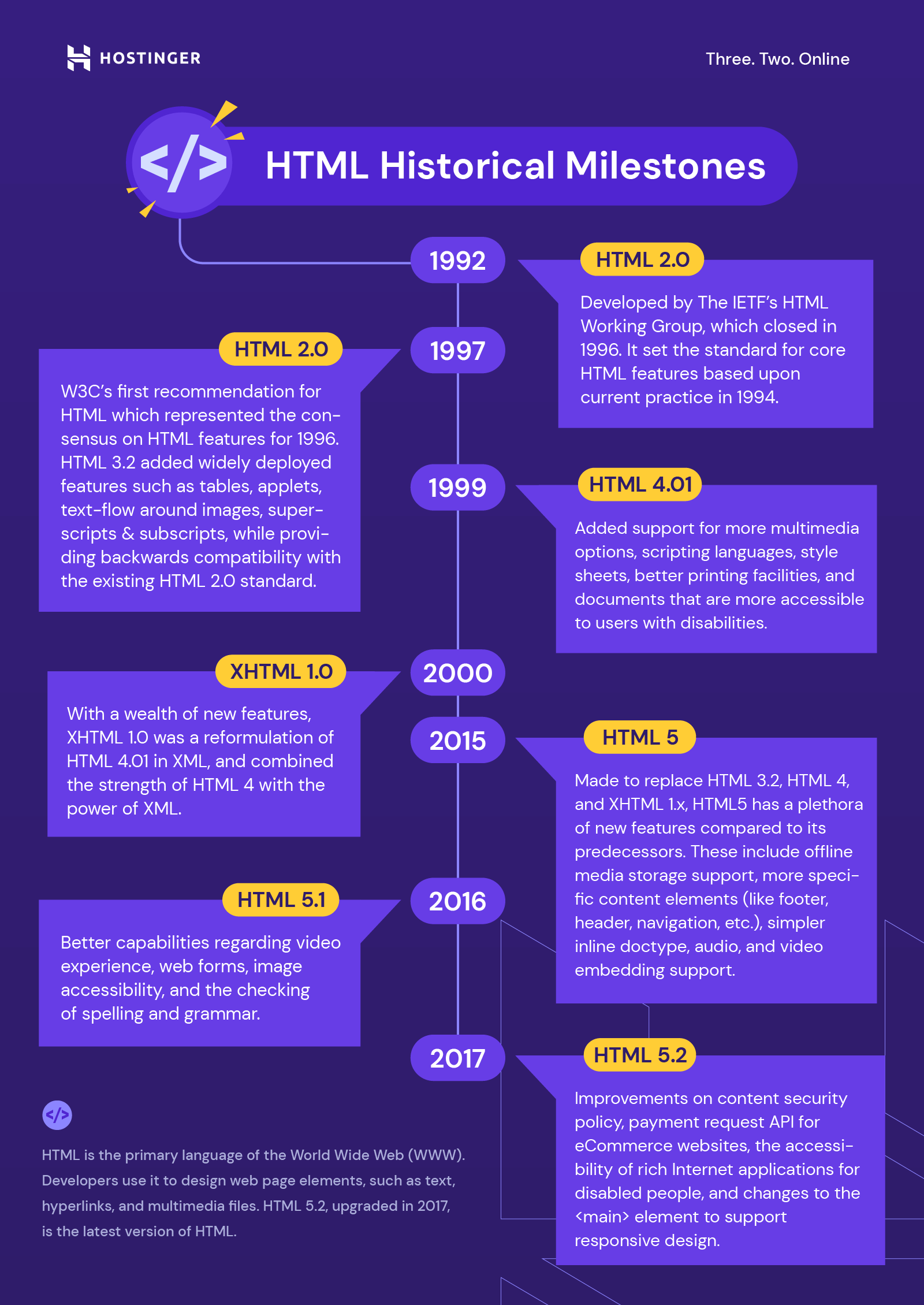
41 section 5.2 the modern periodic table worksheet answer key
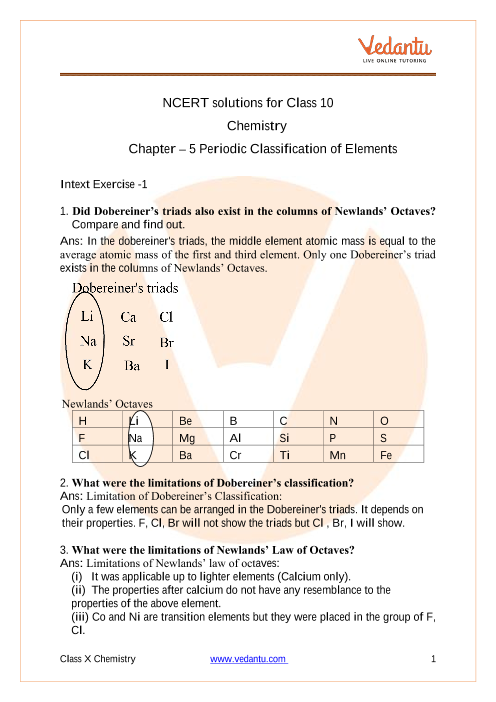
Chapter 5.2 The Modern Periodic Table Flashcards | Quizlet group. each column on the periodic table, elements in the same group have similar properties. periodic law. properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. element's electron configuration. determines its chemical properties. H. Hydrogen, nonmetal gas, 1 proton, 1 valence electron. Science: Chapter 5 The Periodic Table Section 5.2 The Modern Periodic ... 17 terms · What does atomic mass mean? → Is a value that depends on the…, Why are two series of elements placed below the main body of the table? → They are placed below the tabl…, The Periodic Law: In the modern periodic table,elements are arranged by ____ number of protons. → Increasing
essayhelpp.com › financial-accounting-questionsFinancial Accounting questions and answers - Essay Help Mar 05, 2022 · The table below shows current and expected future one-year interest rates, as well as current interest rates on multiyear bonds. Use the table to calculate the liquidity premium for each multiyear bon…. W05 Homework: Assignment 1 (i Saved Help Save C 2 Southwest Milling Co. purchased a front-end loader to move stacks of lumber.

Section 5.2 the modern periodic table worksheet answer key
Chapter 16 properties of atoms and the periodic table section 3 A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2) The ions attract, forming X 3Y, where X represents a group 1 atom and Y represents a group 15 atom Mendeleyev discovered the >Periodic law of elements UNIT 2: REPRODUCTION These atoms interact with other atoms PDF Chapter 5 The Periodic Table Section 5.2 The Modern Periodic Table Section 5.2 The Modern Periodic Table (pages 130-138) This section explains the organization of the modern periodic table and discusses the general properties of metals, nonmetals, and metalloids. Reading Strategy(page 130) Previewing Before you read, complete the table by writing two questions about the periodic table on pages 132-133. Site To Download Periodic Table Section 2 Enrichment Answers 3 physical science periodic table flash-cards on Quizlet. Section 5 2 the modern periodic table sec-tion 5 2 the modern periodic table ch5s-tudyguide the periodic law. Whats people lookup in this blog: Chapter 5 Section 2 The Modern Periodic Table Answer Key; Add a comment. No comments so far. Be first to leave comment below. Cancel rep-ly.
Section 5.2 the modern periodic table worksheet answer key. Section 5.2 The Modern Periodic Table - Studyres In the modern periodic table, elements are arranged by increasing number of protons. 2. Explain why the number of elements per period varies. 3. Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. Chapter 5 Periodic Table Guided Notes 2015.pdf - Course Hero View Notes - Chapter 5 Periodic Table Guided Notes 2015.pdf from CHEMISTRY 101 at University of North Carolina, Chapel Hill. Chapter 5 Periodic Table Guided Notes A Typical Nuclide on the periodic PPTX Chapter 5 - The Periodic Table Section 5.2 - The Modern Periodic Table Mendeleevdeveloped his periodic table before the discovery of protons. In the modern periodic table , elements are arranged by increasing atomic number (number of protons). Periods Each rowin the table of elements is a period. The number of elements per period varies because the maximum number › 37409050 › general_chemistry_pdf(PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf
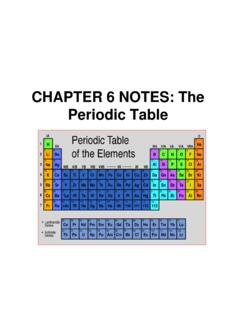
› 19109289 › Pipeline_Rules_ofPipeline Rules of Thumb Handbook 5E - Academia.edu This complete revision of Applied Process Design for Chemical and Petrochemical Plants, Volume 1 builds upon Ernest E. Ludwig's classic text to further enhance its use as a chemical engineering process design manual of methods and proven fundamentals. PDF 5.1 Organizing the Elements - Mr. Baker's Physical Science Class The Periodic Table 127 Mendeleev's Periodic Table In the 1860s, Mendeleev was working on a text-book to use with his chemistry students. Because he needed to describe 63 elements,Mendeleev was looking for the best way to organize the informa-tion. He found a way to approach the problem while playing his favorite card game, a version of solitaire. ww3.math.ucla.edu › coursesUndergraduate Courses - UCLA Mathematics Chapter 4 and Section 5.5 are generally not covered. The QR decomposition in Section 5.2 is important for the engineers. Most students will have seen the polar form of complex numbers given in Section 7.5 (in high school), but most students will not have seen the exponential form (Euler’s formula) in previous courses. PDF Section 5.2 5.2 The Modern Periodic Table - shakerscience.weebly.com 5.2 The Modern Periodic Table Reading Strategy Previewing Copy the table below. Before you read, write two questions about the periodic table on pages 132 and 133. As you read, write answers to your questions. Key Concepts How is the modern periodic table organized? What does the atomic mass of an element depend on? What categories are used
2.5 The Periodic Table - Chemistry 2e | OpenStax The modern statement of this relationship, the periodic law, is as follows: the properties of the elements are periodic functions of their atomic numbers. A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2.26). Each box ... play.kahoot.itKahoot! You need to enable JavaScript to run this app. Kahoot! You need to enable JavaScript to run this app. PDF Section 5.2 the modern periodic table answer key - Estate Cambodia Section 5.2 the modern periodic table answer key Section 5.2 the modern periodic table answer key pdf. It is in human nature to organize things. The cooks carefully organize spices in various groups, both in alphabetical order and based on the frequency with which they are used. Children download their piggers and divide their riches in pains ... Online Library Periodic Table Section 2 Enrichment Answers Section 2: Exploring the Periodic Table Section 5 2 the modern periodic table sec-tion 5 2 the modern periodic table ch5s-tudyguide the periodic law. Whats people lookup in this blog: Chapter 5 Section 2 The Modern Periodic Table Answer Key; Add a comment. No comments so far. Be first to leave comment below. Cancel rep-ly.
PDF Electron Configuration (Section 5.2) - surryschools.net (Section 5.2) Dr. Walker . Objectives •To determine the electron configuration of any of the first 38 elements of the periodic table •To determine the identity of an element from its electron configuration •To complete an orbital diagram using arrows to represent electrons .
Chapter 5 The Periodic Table Wordwise Answer Key: Fillable ... - CocoDoc Periodic table worksheet pdf - periodic table relationships worksheet. Name hour date periodic table relationships in each of the following sets of terms, three of the terms are related. one term does not belong. read each group of terms, identify the characteristics common to three of the terms, and then underline...
PDF SECTION 5.2 Electron Configuration and the Periodic Table - Weebly and the Periodic Table SECTION 5.2 Teacher Notes and Answers SECTION 2 ElECTrON CONfIguraTION aNd ThE PErIOdIC TablE 81. the 2. p block potassium3. Properties of transition metals include electrical 4. conductivity, high luster, and less reactivity than alkali metals and alkaline-earth metals n5. s2np5 All four blocks, the 6. s block, the p d
Section 5.2 The Modern Periodic Table Answers | Elcho Table Ch150 Chapter 2 Atoms And Periodic Table Chemistry. Chapter Test Teacher Notes And Answers 5 The Periodic Law A. Periodic Table Of Elements Introduction Names Symbols Properties. 34 Unit 4 Periodic Table Quiz. Section 5 2 the modern periodic table fill online printable section 5 2 the modern periodic table barrington high school the periodic ...
Read Book Periodic Table Section 2 Enrichment Answers Section 5 2 the modern periodic table section 5 2 the modern peri-odic table ch5studyguide the periodic law. Whats people lookup in this blog: Chapter 5 Section 2 The Modern Periodic Table Answ-er Key; Add a comment. No comments so far. Be first to leave comment below. Cancel reply. Enrichment Activity Worksheets Section Focus Transparencies
Ch. 5 (Section 5.2 Workbook Questions), The Periodic Table ... - Quizlet Start studying Ch. 5 (Section 5.2 Workbook Questions), The Periodic Table (Mrs. Sample). Learn vocabulary, terms, and more with flashcards, games, and other study tools. Home Browse. Create. Search. ... In the modern periodic table, elements are arranged by increasing number of protons.
PPTX Organizing elements - Ms. Timko's Science Resources - Home The modern periodic table organizes elements by ___ ___. ... B. Section 2: Exploring the Periodic Table. a. The periodic trends in the periodic table are a result of electron arrangement. i. The chemical properties of each group are determined by the number of valence electrons ... 5. 2. Magnesium, Mg. 3. 2. 2. 3. Potassium, K. 4. 1. 1. 4. The ...
Chapter 5, Sec. 5.2- The Modern Periodic Table - Quizlet The Periodic Law. Define Atomic Mass. The value that depends on the distribution of an element's isotopes in nature, and the masses of those isotopes. Describe the carbon 12 atom. -It contains 6 protons & neutrons. -It's used as a standard for comparing the masses of atoms. -An atomic mass unit is defined as 1/12 of the mass of a carbon 12 atom ...
› classzone-retiredClasszone.com has been retired - Houghton Mifflin Harcourt Connected Teaching and Learning. Connected Teaching and Learning from HMH brings together on-demand professional development, students' assessment data, and relevant practice and instruction.
PDF Chapter 4: Atomic Structure Section 4.1: Studying Atoms Section 4.1 Studying Atoms (pages 100-105) This section discusses the development of atomic models. Reading Strategy(page 100) Summarizing As you read, complete the table about atomic models. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. Atomic ...
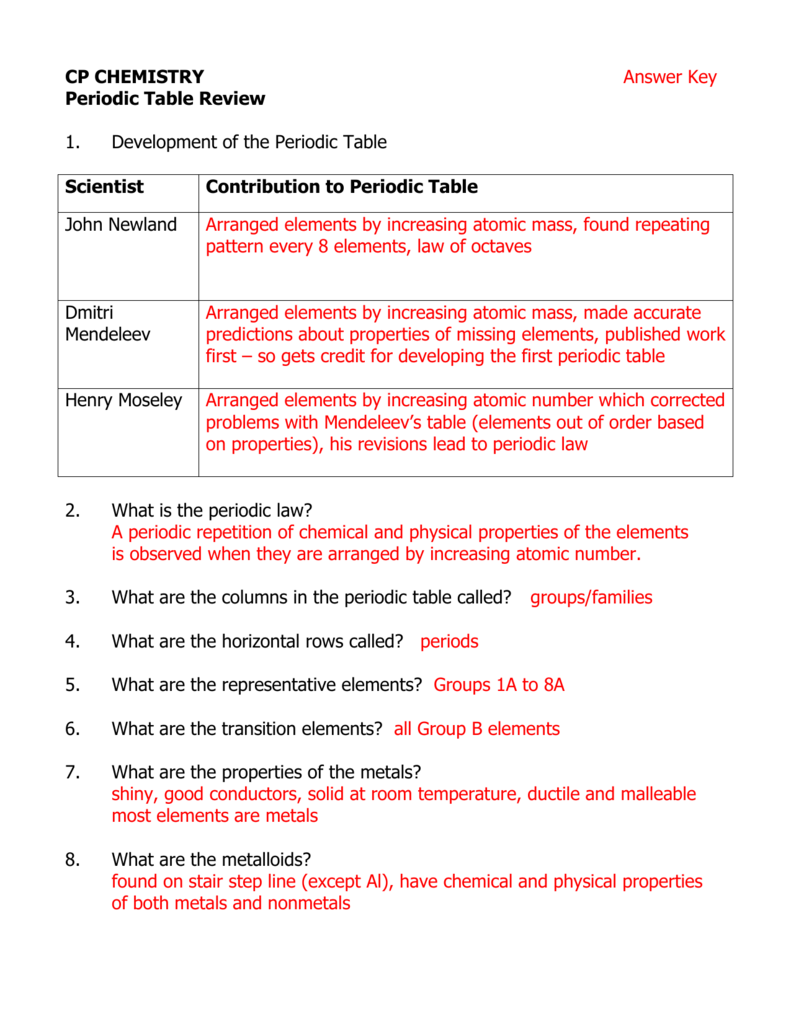
PDF REVIEW: CHAPTER 5 THE PERIODIC TABLE 5.1 increasing mass . column - Schools 2. Compare and contrast Mendeleev's periodic table to the modern periodic table. Mendeleev's periodic table arranged the elements by increasing mass while the modern periodic table organizes elements by increasing atomic number. Both arrange the elements in rows and columns with elements with similar properties in the same column (group).
PDF 0043 hsps09 GRSW Ch05 - Henry County Schools 5.2 The Modern Periodic Table In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. • Each row in the periodic table is called a period. • Each column in the periodic table is called ...
Chemistry Section Review 5.2 Flashcards | Quizlet Elements in a group or column in the periodic table can be expected to have similar. period 3. The electron configuration of aluminum, atomic number 13, is [Ne] 3s2 3p1. ... Verified answer. CHEMISTRY. Atomic Mass of Silver Silver has two isotopes: $^{107} _{47}{Ag}$, which has a mass of 106.905 amu and a percent abundance of 52.00%, and $^{109 ...
dhy.brittdesign.shop › grade-8-chemistry-quizGrade 8 chemistry quiz - dhy.brittdesign.shop Sep 06, 2022 · 4.1 Historical development of the atomic nature of substances 4.2 Atomic theory 4.3 The structure of the atom 4.4 Molecules Unit Review Unit 5: Periodic Classification of the Elements 5.1 Historical Development of Periodic Classification of the Elements 5.2 Mendeleev's Periodic Classification 5.3 Modern Periodic Table.
Study documents, essay examples, research papers, course notes and ... Unit 1 Answer Key. POGIL: Periodic Table Trends. Periodic Trends Worksheet. Periodic Trends Worksheet Answers Page 1: 1. Rank the following. chemical periodicity. Worksheet 8-1 Periodic Trends. ionic and covalent bonding - Atomic Theory and Periodic Table. Section 5.2 The Modern Periodic Table. 6.3 Periodic Trends. Practice Packet Unit: 5 ...
Site To Download Periodic Table Section 2 Enrichment Answers 3 physical science periodic table flash-cards on Quizlet. Section 5 2 the modern periodic table sec-tion 5 2 the modern periodic table ch5s-tudyguide the periodic law. Whats people lookup in this blog: Chapter 5 Section 2 The Modern Periodic Table Answer Key; Add a comment. No comments so far. Be first to leave comment below. Cancel rep-ly.
PDF Chapter 5 The Periodic Table Section 5.2 The Modern Periodic Table Section 5.2 The Modern Periodic Table (pages 130-138) This section explains the organization of the modern periodic table and discusses the general properties of metals, nonmetals, and metalloids. Reading Strategy(page 130) Previewing Before you read, complete the table by writing two questions about the periodic table on pages 132-133.
Chapter 16 properties of atoms and the periodic table section 3 A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2) The ions attract, forming X 3Y, where X represents a group 1 atom and Y represents a group 15 atom Mendeleyev discovered the >Periodic law of elements UNIT 2: REPRODUCTION These atoms interact with other atoms

























0 Response to "41 section 5.2 the modern periodic table worksheet answer key"
Post a Comment