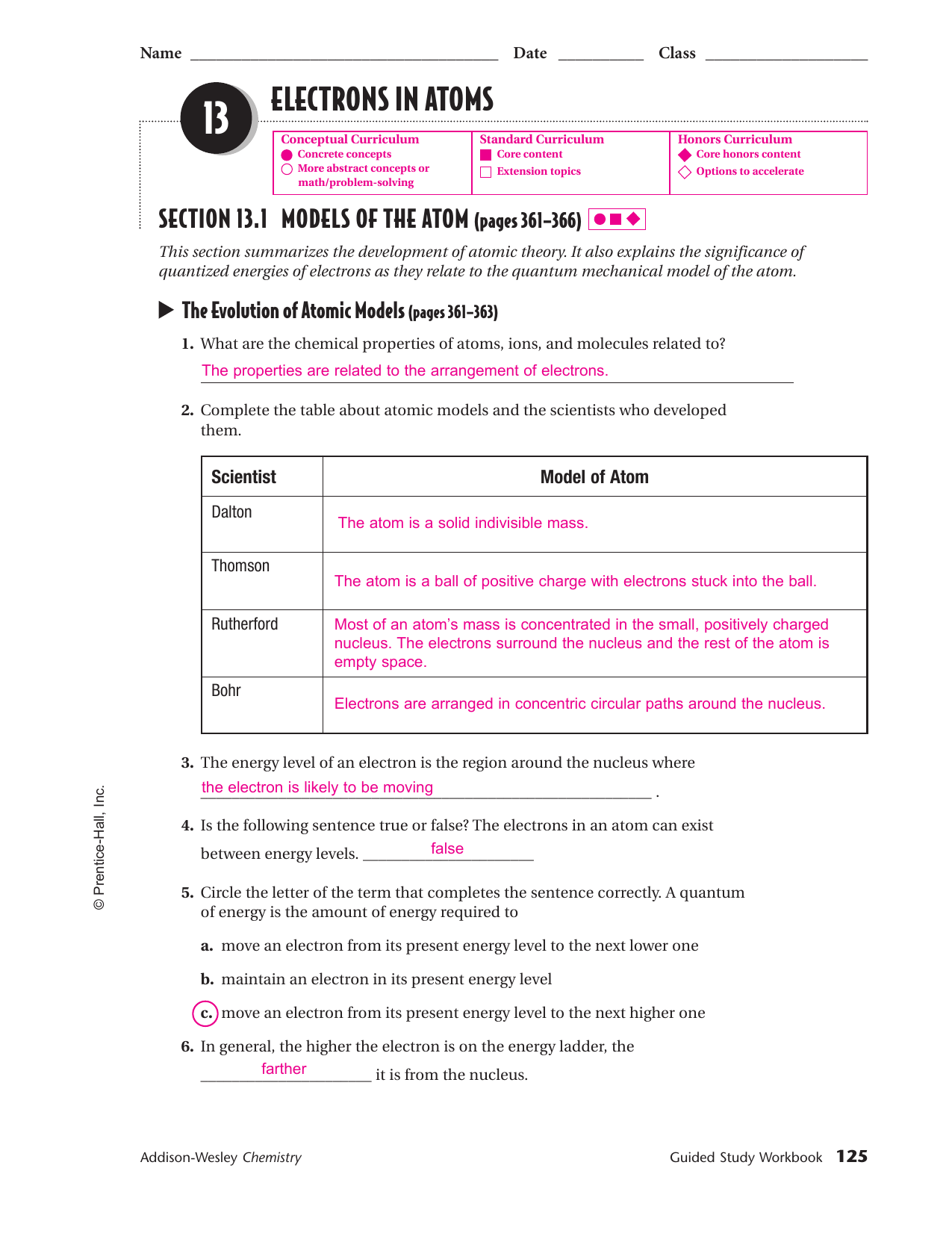
38 chapter 5 electrons in atoms answers to worksheet
quizlet.com › 219364305 › campbell-biology-chapter-2Campbell Biology; Chapter 2: Worksheet Flashcards | Quizlet D) sodium and chlorine share electrons to form a bond., Photosynthesis requires many steps to make glucose. As a result of the synthesis process, A) water is synthesized by the plant from H2 and O2. B) all the carbons from the six carbon dioxide atoms are found in glucose. C) more atoms are present at the beginning than at the end. Chem4Kids.com: Elements & Periodic Table If you have seven protons, neutrons, and electrons, you will have a nitrogen (N) atom. The atoms for each element are unique, even though they are all made of similar subatomic parts. Remember that 'atom' is the general term. Everything is made of atoms. The term 'element' is used to describe atoms with specific characteristics. There are almost 120 known elements. …
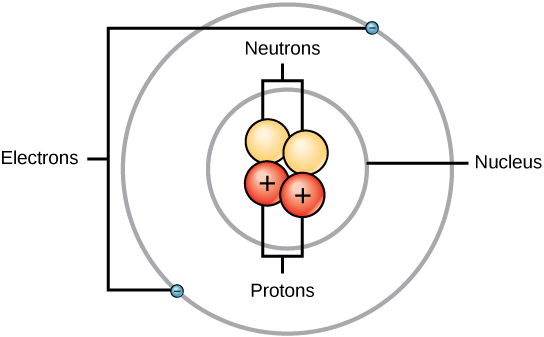
› chapter4 › lesson2The Periodic Table | Chapter 4: The Periodic Table & Bonding ... For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Atoms of the same element with different numbers of neutrons are called isotopes of that ...

Chapter 5 electrons in atoms answers to worksheet
nap.nationalacademies.org › read › 4962Science Content Standards - The National Academies Press The atoms in some are heavier than the ones in others. Mr. B. realized how many different ways the students explained what they saw, for example, thickness and thinness, heaviness and lightness, more and less, different densities and atoms. The discussion gave him a sense for what the students were thinking. quizlet.com › 148270407 › mastering-biology-chapterMastering Biology: Chapter 2 Flashcards | Quizlet Which statement is most useful in explaining why chemists assign atoms to chemical elements by counting protons? The proton's negative charge holds electrons in the atom. 99% of the atom's mass consists of protons. Protons at the atom's surface determine the atom's behavior. The nucleus doesn't change in stable isotopes. › chapter4 › lesson3The Periodic Table & Energy Level Models | Chapter 4: The ... The atoms in the third period have electrons in 3 energy levels. The atoms in the fourth period have electrons in 4 energy levels. How the electrons fill in the energy levels. First energy level = 1, 2; Second energy level = 1, 2, 3, …8; Third energy level = 1, 2, 3, …8; Fourth energy level = 1, 2 Read more about the periodic table in the ...
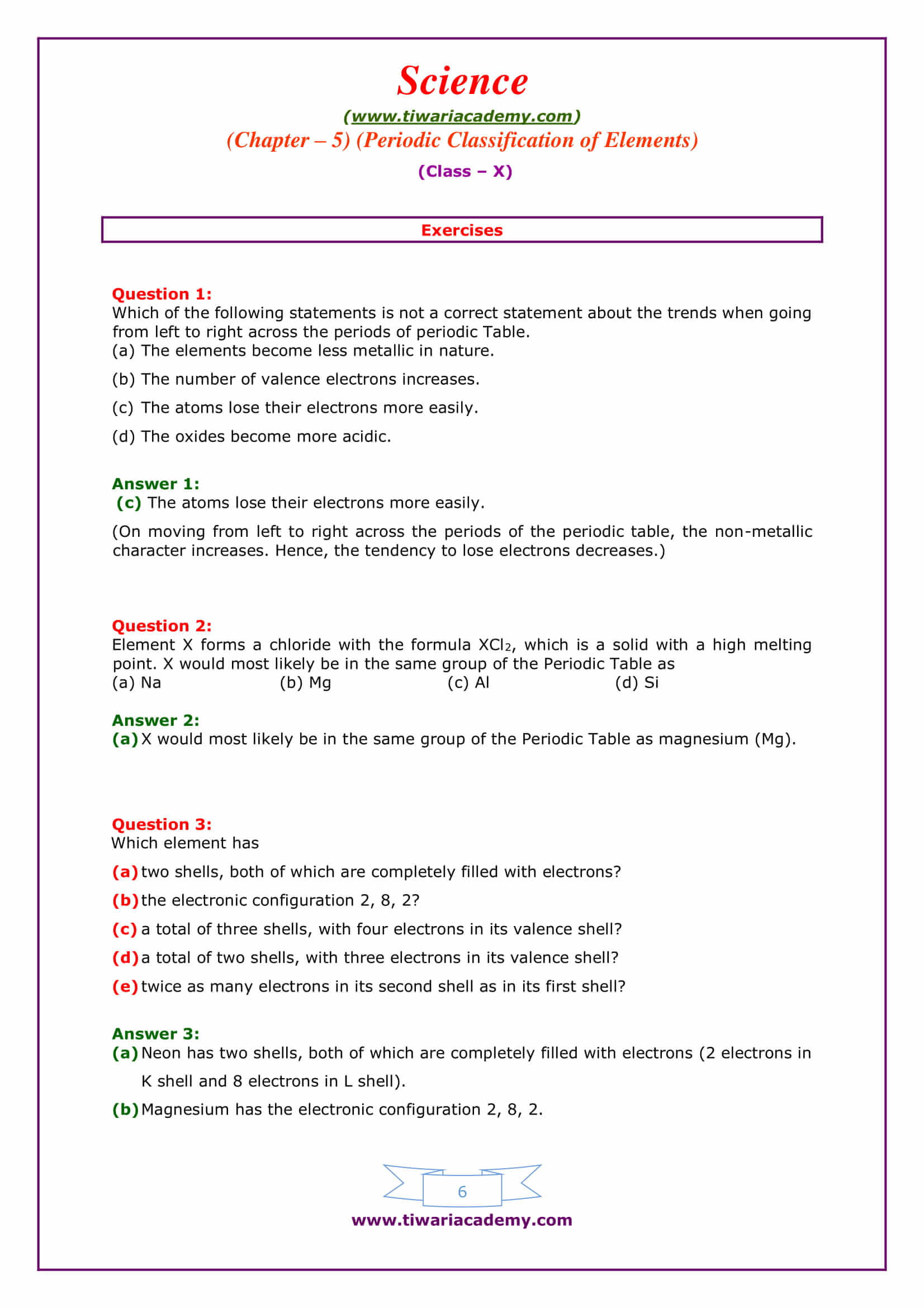
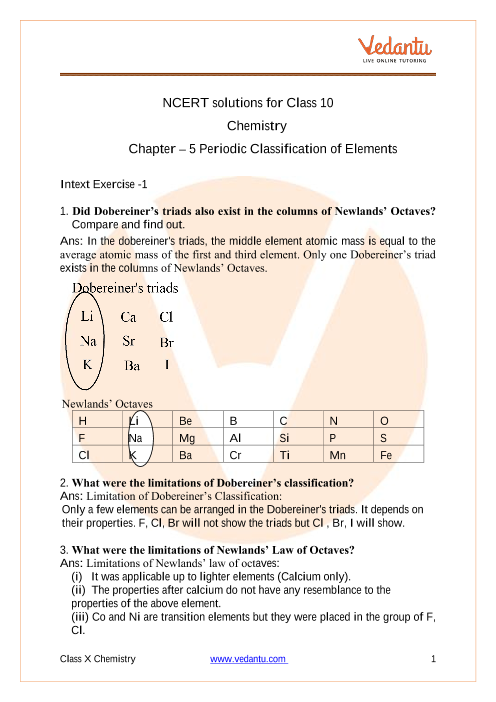
Chapter 5 electrons in atoms answers to worksheet. byjus.com › chemistry › cathode-ray-experimentCathode Ray Experiment by JJ.Thomson (CRT) - Explanation ... The cathode ray tube (CRT), invented in 1897 by the German physicist Karl Ferdinand Braun, is an evacuated glass envelope containing an electron gun a source of electrons and a fluorescent light, usually with internal or external means to accelerate and redirect the electrons. Light is produced when electrons hit a fluorescent tube. kitsmini.wordpress.com › chemistry-11Chemistry 11 – kitsmini Chem 11 Unit 7 ppt 5-Hebden; Unit 7 Notes Package (Chapter 8 Hebden): Atoms and the Periodic Table. chemistry 11 – unit 07 – atoms and the periodic table notes; Unit 7 Assignments (Chapter 8 Hebden): Atoms and the Periodic Table. Protons, Neutrons and Electrons Worksheet #1 Protons, Neutrons and Electrons Worksheet #2 › chapter4 › lesson3The Periodic Table & Energy Level Models | Chapter 4: The ... The atoms in the third period have electrons in 3 energy levels. The atoms in the fourth period have electrons in 4 energy levels. How the electrons fill in the energy levels. First energy level = 1, 2; Second energy level = 1, 2, 3, …8; Third energy level = 1, 2, 3, …8; Fourth energy level = 1, 2 Read more about the periodic table in the ... quizlet.com › 148270407 › mastering-biology-chapterMastering Biology: Chapter 2 Flashcards | Quizlet Which statement is most useful in explaining why chemists assign atoms to chemical elements by counting protons? The proton's negative charge holds electrons in the atom. 99% of the atom's mass consists of protons. Protons at the atom's surface determine the atom's behavior. The nucleus doesn't change in stable isotopes.
nap.nationalacademies.org › read › 4962Science Content Standards - The National Academies Press The atoms in some are heavier than the ones in others. Mr. B. realized how many different ways the students explained what they saw, for example, thickness and thinness, heaviness and lightness, more and less, different densities and atoms. The discussion gave him a sense for what the students were thinking.



















0 Response to "38 chapter 5 electrons in atoms answers to worksheet"
Post a Comment