41 5.1 models of the atom worksheet answers
› indexPHSchool.com Retirement–Prentice Hall–Savvas Learning Company PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support. [FREE] 5.1 Models Of The Atom Quiz Answers | latest Answers to Review Questions for Atomic Theory Quiz #1 nucleus of the atom and that electrons are flying in a random electron cloud around the nucleus in Rutherford's model, the atom is mostly empty space and this is where the electrons are found 19. Compare and contrast the models of the atom that were proposed by Rutherford and Bohr.
5.1 models of the atom worksheet answer - ecoland-crimea.ru Rutherford's model fails to explain why objects change color when heated. Chapter 5 Electrons In Atoms Workbook Answers Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2 5.1 models of the atom worksheet answer 8 5.1 models of the atom worksheet answer and 8.)

5.1 models of the atom worksheet answers
issuu.com › cupeducation › docsCambridge IGCSE Chemistry Coursebook (fourth edition) - Issuu Jun 09, 2014 · A simplified version of Bohr’s theory of the arrangement of electrons in an atom can be summarised as follows (see also Figure 2.33): Electrons are in orbit around the central nucleus of the ... 51 Models Of The Atom Worksheet Answers - Nidecmege 51 models of the atom worksheet answers. Probability electrons must have a certain minimum amount of energy called a quantum of energy in order to move from one energy level to the next higher energy level. ... Homework On Models Of Atoms Forum H Section 5 1 Models Of The Atom 5 1 Models Of The Atom Section Review Objectives 5 3 Physics And The ... 5.1 Models Of The Atom Answers - acscu.net [GET] 5.1 Models Of The Atom Answers | HOT! Question 5 (1 point) In the Bohr model of the atom, for a short arrow that points in toward the center/nucleus (e.g. from n = 2 to n = 1), select all of the following statements that are true. The arrow pointing in means that the electron is losing energy.
5.1 models of the atom worksheet answers. PDF 5.1 models of the atom answer key how electrons were arranged within atoms. 51 models of the atom worksheet answers. 51 light and quantized energy 117. ... Section Review 4 5 6 Notes 2015 Print Pages 1 50 Text Version Fliphtml5 5 1 Models Of The Atom Worksheet Answers Match Problems Atom Model Quiz Proprofs Quiz Chemistry Teacher Book Chapter 5 1 Electron Configuration Atomic 5 ... edu.rsc.org › resources › hydrocarbons-organicHydrocarbons – organic chemistry worksheets | 14–16 Oct 22, 2020 · 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes 4.1.1.1 Atoms, elements and compounds Chemical reactions can be represented by word equations or equations using symbols and formulae. ️Models Of Atoms Worksheet Answers Free Download| Qstion.co Atomic structure worksheet pdf answers. Bohr atomic models worksheet answers. Models of atoms worksheet answers 5.1 models of the atom worksheet answers. Atoms isotopes and ions teacher answer key in this laboratory activity you will use chips and the information in a reference sheet to make models of atoms isotopes and ions of various elements. kitchingroup.cheme.cmu.edu › pycse › pycsepycse - Python3 Computations in Science and Engineering x = [0 0.5 1 1.5 2]; y = [0 0.1250 1.0000 3.3750 8.0000]; and you want to integrate this from x = 0.25 to 1.75. We do not have data in those regions, so some interpolation is going to be needed. Here is one approach.
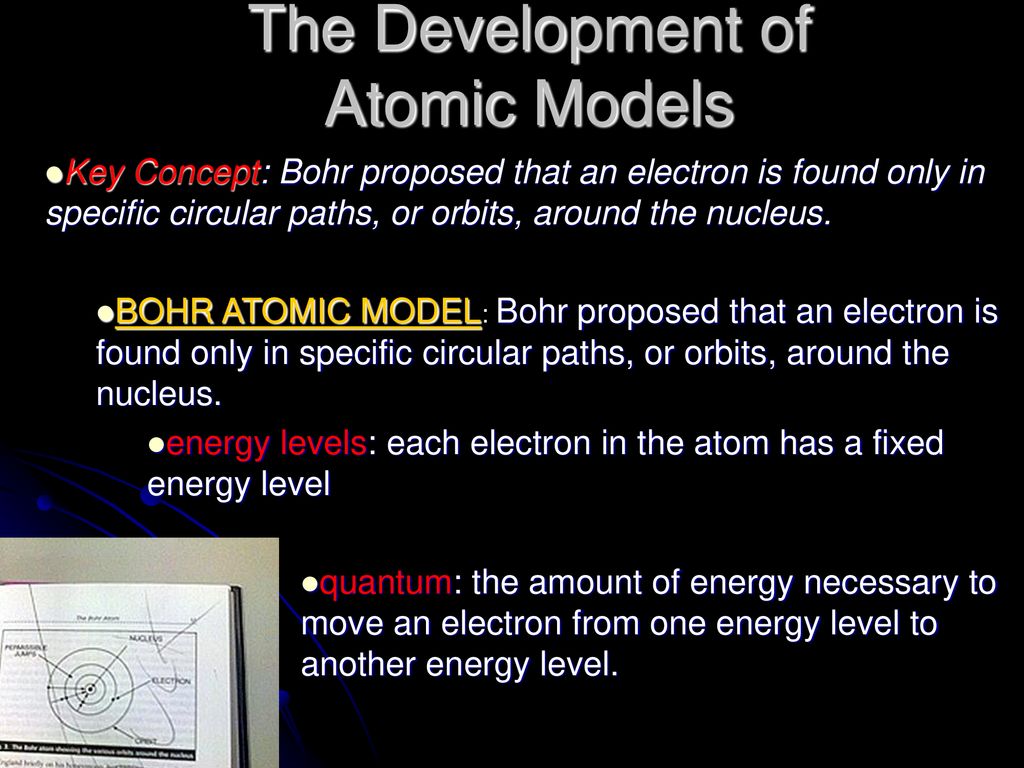
Worksheet 5.1 - Models of the Atom.pdf - Course Hero _____ The Bohr Model of the atom says that: a. Electrons exist at different energy levels in orbit around the nucleus. b. Electrons can move between energy levels when they gain or lose energy. c. Electrons cannot exist in between an energy level. d. All of the above. 2. _____Which of the following best fits Thomson's model of the atom? a. 5.1 Models of The Atom Answer Key WONDERS YOUR TURN PRACTICE BOOK GRADE 1 ANSWER KEY . Question Correct Answer Content Focus CCSS Complexity 14 G Main Idea and Key Details RI32 DOK 2 15 B Main Idea and Key Details RI32 DOK 2 16 I Context Clues L34a DOK 2 Listen to the highly anticipated memoir A Promised Land. Unit 11 Unit One Week One. Answers to Wonders Week One. 5.1 Models of the Atom Section Review Flashcards | Quizlet The electron probability clouds for atomic orbitals are spherical in shape Sometimes True (ST) The number of sublevels in an energy level is equal to the square of the principal quantum number of that energy level Never True (NT) The maximum number of electrons that can occupy the fourth principal energy level of an atom is 32 Always True (AT) › 34707649 › Managerial_EconomicsManagerial Economics Textbook - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax The chemical equation representing this process is provided in the upper half of Figure 4.2, with space-filling molecular models shown in the lower half of the figure. Figure 4.2 The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by a chemical equation using formulas (top). PDF Models of the atom worksheet answers It was Ernest Rutherford who, in 1911, used this discovery to revise the model of the atom. Two other models proposed for the atom were the cubic model and the Saturnian model. In the cubic model, the electrons were imagined to lie at the corners of a cube. In the Saturnian model, the electrons were imagined to orbit a very big, heavy nucleus. › document › 524079710Cambridge IGCSE Chemistry Coursebook 4th Edition | PDF ... Contents iii 10 Organic chemistry 252 Answers to questions 326 10.1 The unique properties of carbon 253 10.2 Alkanes 254 Glossary 336 10.3 Alkenes 257 Appendix: The Periodic Table 346 10.4 Hydrocarbon structure and isomerism 259 10.5 Chemical reactions of the alkanes 262 Index 347 10.6 Chemical reactions of the alkenes 263 Acknowledgements 353 ... 5.1 models of the atom Flashcards | Quizlet Summarize the development of atomic theory ( short answer ) 1. Dalton proposed that matter was made of indestructible particles called Atoms 2. Thompson's proposed that the atomic model in which negatively charged electrons were embedded in a positively charged Mass. 3. Rutherford discovered that atoms are mainly empty space 4.
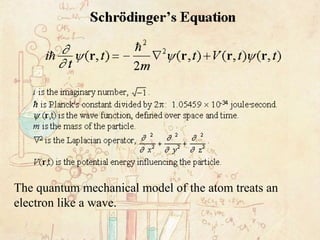
PDF SECTION 5.1 MODELS OF THE ATOM (pages 127-132) The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. _____ Atomic Orbitals (pages 131-132) 8. Circle the letter of the term that correctly answers this question. Which name describes the major energy levels of electrons? a. atomic orbitals b. quantum mechanical numbers c. quantas d. ...
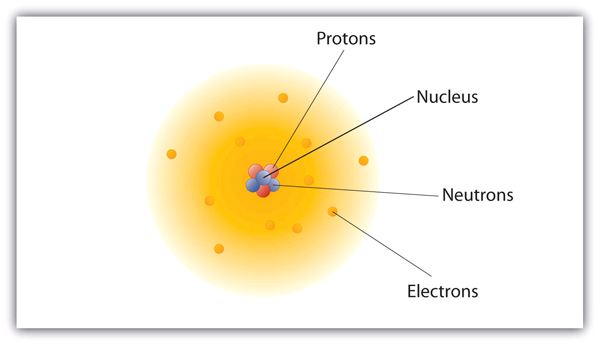
PDF Models of the atom worksheet answer key Another way of thinking about this model was that the atom was seen to be like a mini solar system where the electrons orbit the nucleus like planets orbiting around the sun. A simplified picture of this is shown alongside. This model is sometimes known as the planetary model of the atom. Figure 4.4: Rutherford's model of the atom. There were ...
PDF 5.1 Models of the Atom - Weebly Models of the Atom > The Quantum Mechanical Model The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. 5.1 . 11/18/14 8
PDF 5.1 Models of the Atom 5 Section 5.1 Models of the Atom 127 5.1 Models of the Atom Aeronautical engineers use wind tunnels and scale models to simulate and test the forces from the moving air on each proposed design. The scale model shown is a physical model. However, not all models are physical. In fact, several theoretical models of the atom
5.1 Models Of The Atom Answers - acscu.net [GET] 5.1 Models Of The Atom Answers | HOT! Question 5 (1 point) In the Bohr model of the atom, for a short arrow that points in toward the center/nucleus (e.g. from n = 2 to n = 1), select all of the following statements that are true. The arrow pointing in means that the electron is losing energy.
51 Models Of The Atom Worksheet Answers - Nidecmege 51 models of the atom worksheet answers. Probability electrons must have a certain minimum amount of energy called a quantum of energy in order to move from one energy level to the next higher energy level. ... Homework On Models Of Atoms Forum H Section 5 1 Models Of The Atom 5 1 Models Of The Atom Section Review Objectives 5 3 Physics And The ...
issuu.com › cupeducation › docsCambridge IGCSE Chemistry Coursebook (fourth edition) - Issuu Jun 09, 2014 · A simplified version of Bohr’s theory of the arrangement of electrons in an atom can be summarised as follows (see also Figure 2.33): Electrons are in orbit around the central nucleus of the ...































0 Response to "41 5.1 models of the atom worksheet answers"
Post a Comment