41 specific heat worksheet answer key
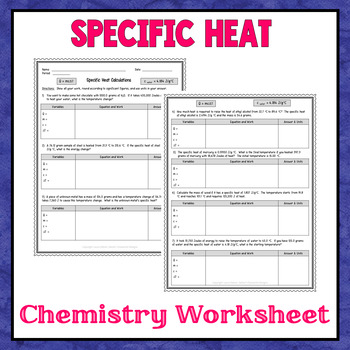
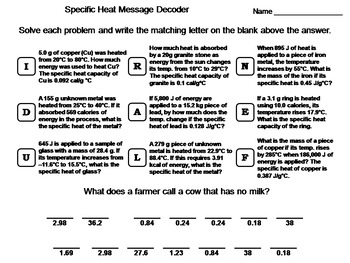
Specific Heat Calculations Worksheet Solution Key - Docsity Apr 20, 2021 ... Download Exercises - Specific Heat Calculations Worksheet Solution Key | Baker College | solved practice test on specific heat chemistry ... specific heat problems answer key.pdf - ISD 622 Specific Heat Worksheet. Rey. Cp = q/mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat ...
Specific Heat Worksheet KEY. Specific Heat Worksheet. Name (in ink):. C = q/mAT, where q = heat energy, ... Answers are provided at the end of the worksheet without units.

Specific heat worksheet answer key
1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ... Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the. Specific Heat Worksheet q=(mass)(Csp)(AT) Specific Heat Worksheet ... What is the specific heat of a substance that absorbs 2.5 x 103 joules of heat when ... 0°C? Explain your answer quantitatively. 16.1 specific heat practice part 2 answer key - StuDocu - StuDocu This is about delta heat. q=mc(delta)T. Formula 0.404 cal 2.00 104 cal 0.1960 kcal 105 2.00 1820 0.449 50.8 10) 0.424 16.1 specific heat practice make the.
Specific heat worksheet answer key. Specific Heat Worksheet If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to cause this rise in temperature. A total of 54.0 joules of heat are ... Worksheet- Introduction to Specific Heat Capacities - LPS Worksheet- Introduction to Specific Heat Capacities ... Step 2: Answer questions ... ***Specific heat capacity = the amount of heat needed to raise the ... Specific heat worksheet Flashcards - Quizlet Study with Quizlet and memorize flashcards containing terms like 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? Calculating Specific Heat Extra Practice Worksheet - TSFX If the specific heat of copper is 390 J/g 0C, what is the change of the copper's temperature? Page 4. Answers. Q = mc∆T, where Q = heat energy, ...
16.1 specific heat practice part 2 answer key - StuDocu - StuDocu This is about delta heat. q=mc(delta)T. Formula 0.404 cal 2.00 104 cal 0.1960 kcal 105 2.00 1820 0.449 50.8 10) 0.424 16.1 specific heat practice make the. Specific Heat Worksheet q=(mass)(Csp)(AT) Specific Heat Worksheet ... What is the specific heat of a substance that absorbs 2.5 x 103 joules of heat when ... 0°C? Explain your answer quantitatively. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy ... Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the.





























0 Response to "41 specific heat worksheet answer key"
Post a Comment