42 oxidation number worksheet with answers
Dec 19, 2021 · In 20.2 oxidation numbers worksheet answers, assigning oxidation numbers worksheet answers, assigning oxidation numbers worksheet answers chemistry if8766, charting oxidation number worksheet answers, oxidation number worksheet pdf answer key
Worksheet Oxidation Numbers Answers payasufo from Oxidation Numbers Worksheet Answers. , source: payasu.info. Periodic Table of Elements on Mars Worksheet for 8th 10th Grade from Oxidation Numbers Worksheet Answers. , source: lessonplanet.com. REDOX lesson 1 Determination of oxidation number AS Chemistry from Oxidation Numbers Worksheet Answers.
Oxidation Number Worksheet with Answers. 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, rules for assigning oxidation numbers worksheet answers, chapter 7 charting oxidation number worksheet answers, assigning oxidation number worksheet answers ...

Oxidation number worksheet with answers
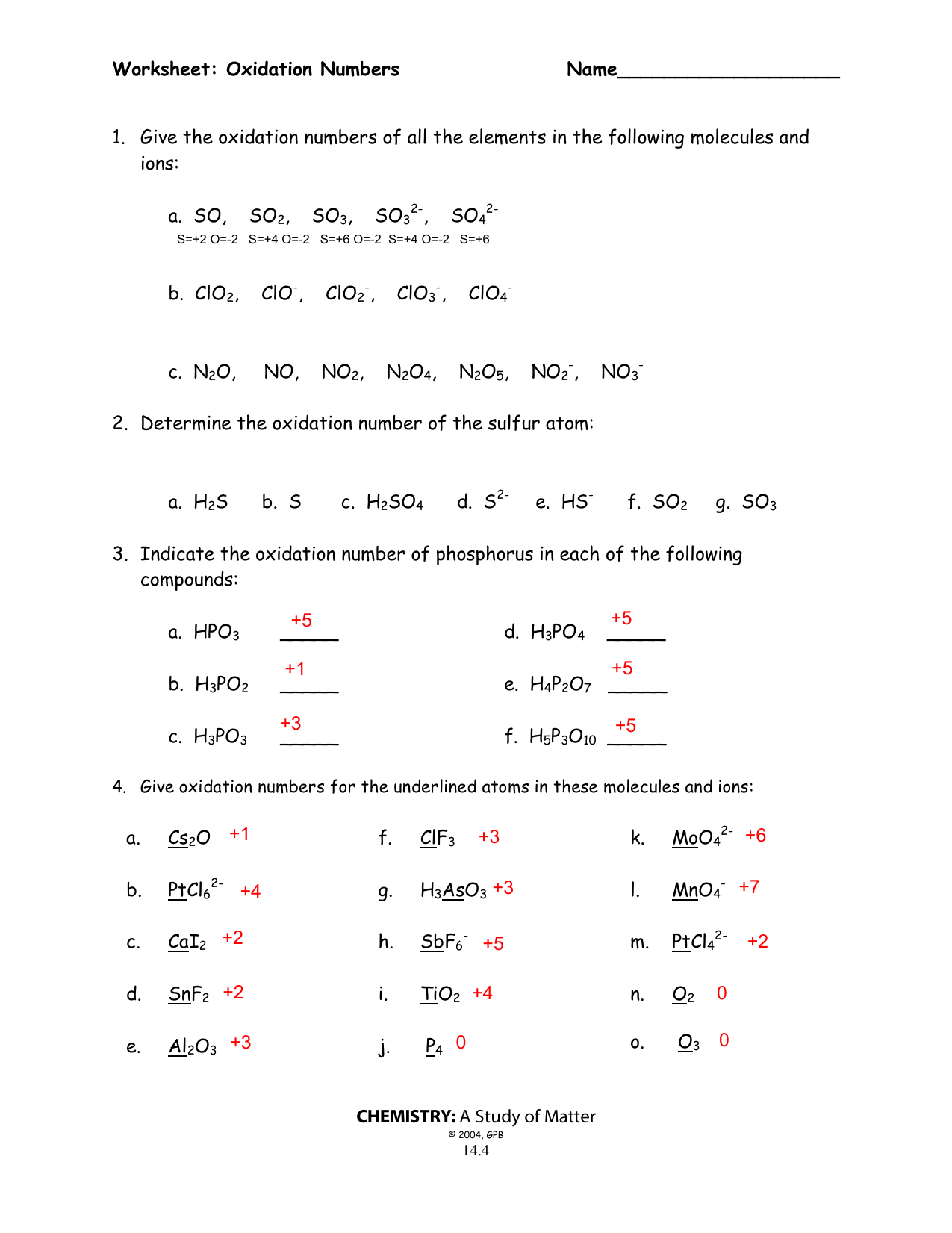
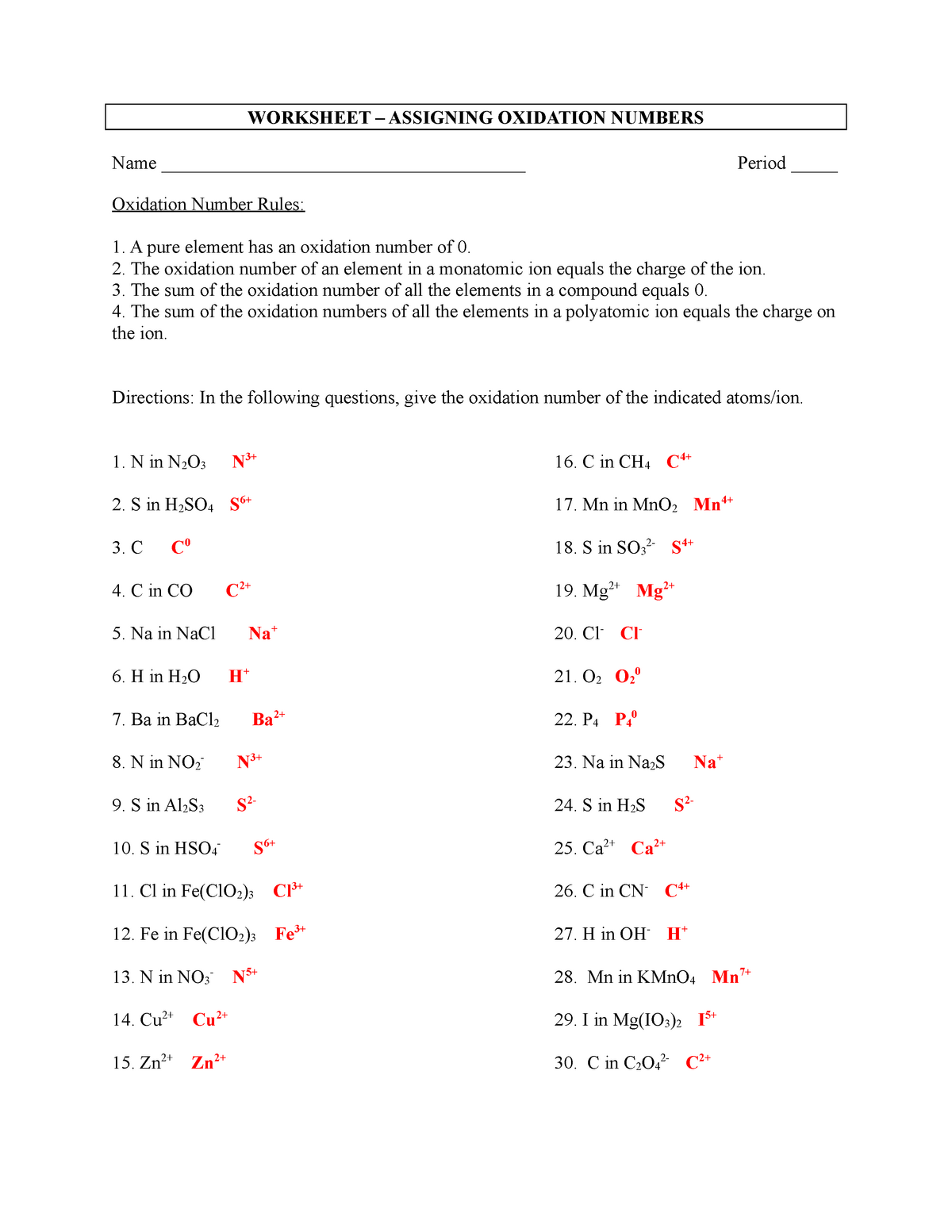
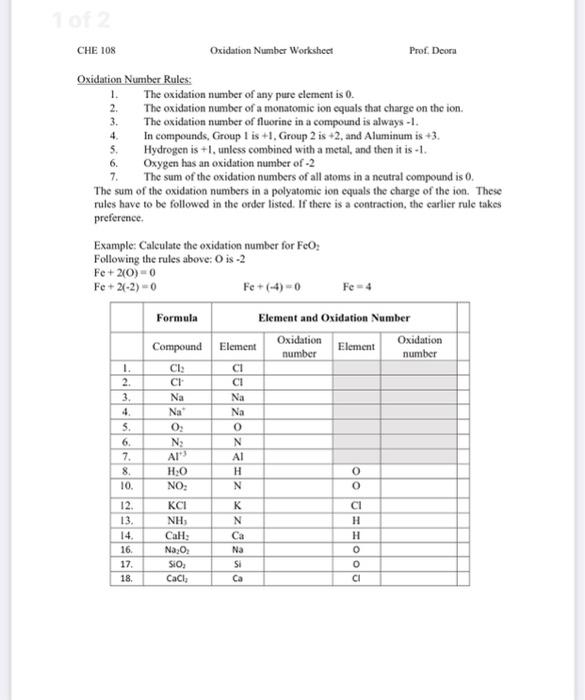
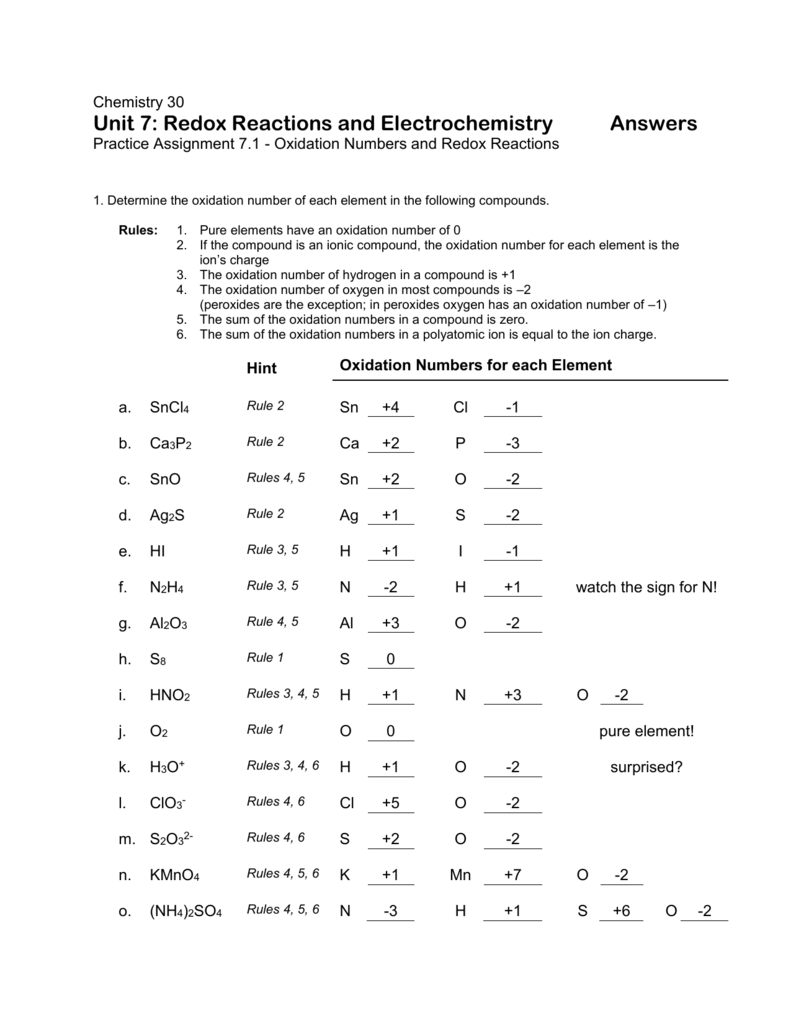
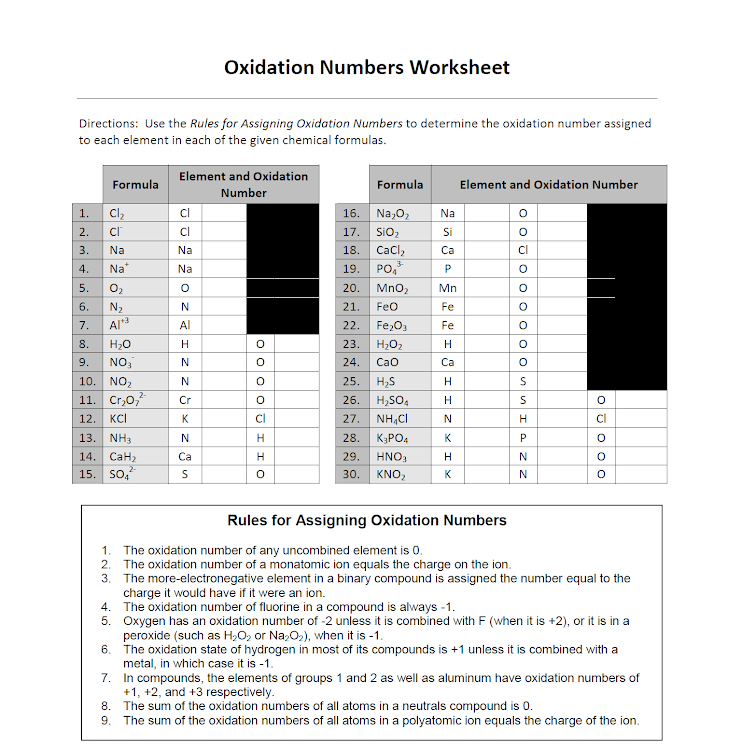
Oxidation Numbers Worksheet Directions: Use the Rules for Assigning Oxidation Numbers to determine the oxidation number assigned to each element in each of the given chemical formulas. Formula Element and Oxidation Number Formula Element and Oxidation Number 1. Cl 2 Cl 16. Na 2 O 2 Na O 2. -Cl Cl 17. SiO 2 Si O 3. Na Na 18. CaCl 2
11. Give the oxidation number of each kind of atom or ion. a. sulfate b. Sn c. S2-d. Fe3+ e. Sn4+ f. nitrate g. ammonium 12. Calculate the oxidation number of chromium in each of the following. a. Cr2O3 b. Na2Cr2O7 c. CrSO 4 d. chromate e. dichromate 13. Use the changes in oxidation numbers to determine which elements are oxidized and which
Oxidation Number Exercise - answers Page 57 Oxidation Number Exercise Do not hand in this work sheet. When you are ready, you will be given an examination over this material. Complete the examination by yourself and hand it in to receive credit. Purpose: This exercise is designed to teach the student how to assign oxidation numbers.
Oxidation number worksheet with answers.
Oxidation and reduction worksheet with answers pdf. Give the oxidation number of each kind of atom or ion. Oxidation reduction worksheet answers 1. 10h 4zn no 3 4zn2 nh 4 3h 2o 3. Multiply one or both of these numbers by appropriate. H is reduced oa. Neither oxidation nor reduction 23. Oxidation number method 1.
ID: 1514798 Language: English School subject: Chemistry Grade/level: 10 Age: 14-15 Main content: Oxidation number Other contents: Add to my workbooks (0) Download file pdf Embed in my website or blog Add to Google Classroom
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq)
Oxidation number worksheet with answers no rating 0 customer reviews. Ptcl 4 2 n. Answer key cio cll nao 5. Brent white created date. N 3 h 1 19. Worksheet will open in a new window. Sio2 si o 3. H 3aso 3 h. Na2o2 na o 2. 7 Readable Language Arts Worksheets 7th Grade In 2020 Chemistry Worksheets Science Notes Element Chart ...
Assigning oxidation numbers worksheet answer key. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. Al s cl2 g o3 g the total oxidation number of a neutral compounds 0 co2 h2o the oxidation number of a monatomic ion is equal to its charge.
Finding oxidation numbers worksheet answers. Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each element in each of the given chemical formulas. Brent white created date. The net charges on all molecules is zero. Oxidation numbers worksheet directions.
For the common polyions know their charges and their names. Microsoft word 14 04 oxidation numbers worksheet doc author. Ptcl 4 2 n. H 3aso 3 h. Download file pdf oxidation numbers worksheet with answers oxidation numbers worksheet with answers when somebody should go to the book stores search creation by shop shelf by shelf it is really ...
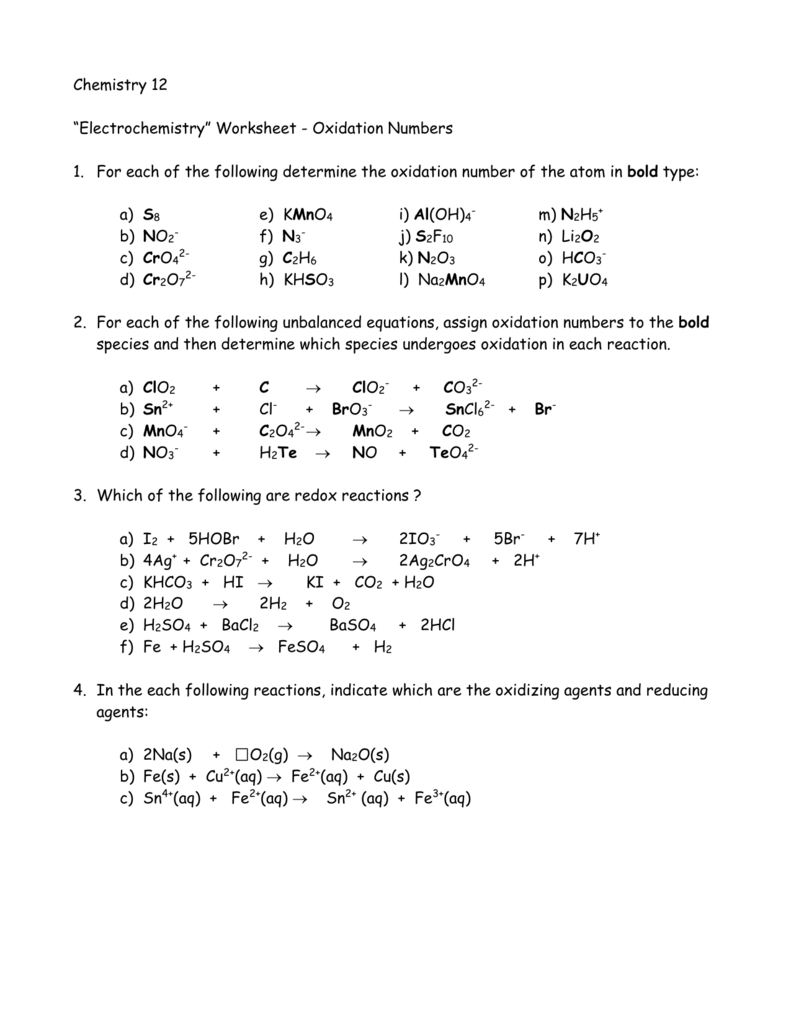
Balancing Redox Equations WorkSheet Oxidation Number Method for Balancing Redox Equations 1. 2. 3. 4. 5. 6. 7. Assign oxidation numbers to all elements and identify ...
State of the change that represents oxidation, reduction or neither. Use oxidation #s. Remember that if the oxidation # increases it means oxidation and when it decreases it mean reduction! 18. MnO 2 → Mn 2O 3 19. NH 3 → NO 2 20. HClO 4 → HCl + H 2O 21. O 2 → O2-22. P 2O 5 → P 4H 10 Determine the oxidation number 23. H 2SO 4 22. HSO 4 ...
Naming Ionic Compounds Worksheet Answer Key. The oxidation number of an element in a monatomic ion equals the charge of the ion. 4th Grade Math Worksheets. 00×102 mL at a temperature of 20. 2 days agoThe answer key helps the candidates to check their numbers of correct answers and wrong answers.
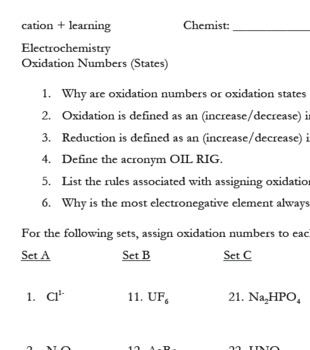
Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
Chemistry Chapter 7 Worksheets —Chemical Formulas and Nomenclature page 1 WORKSHEET 1: Determination of oxidation number or valence number Rules to apply: 1. a. The net charges on all molecules is zero; therefore, the sum of the positive charges equals the sum of the negative charges 2. a.
Oct 10, 2021 · Oxidation numbers and redox reactions 1. Na 2 o 2 na o 2. Oxidation Number Periodic Table Chemistry Worksheets Periodic Table Chemistry Formula element and oxidation number formula element and oxidation number 1. Oxidation numbers worksheet answers. When you are ready you will be given an examination over this material. Determine the oxidation number of […]
Oxidation Number: Definition, Rules & Examples. Worksheet. 1. Which of the following statements is true? Hydrogen can only have one possible oxidation number. The oxidation number of oxygen is ...
Oxidation numbers worksheet answers. The oxidation number of fluorine in a compound is always 1. Na 2 o 2 na o 2. Oxidation numbers worksheet directions. The oxidation number of barium identifying charges in given substances identifying oxidation numbers in given compounds skills practiced.
Aug 28, 2021 · Oxidation reduction worksheet answers 1. H is reduced oa. Assign oxidation numbers to all elements in the reaction 2. Ag no 3 ag no answer. In the reaction mg cl2 mgcl2 the correct half reaction for the. Lose electrons only c. Neither gain nor lose electrons 24. 10h 4zn no 3 4zn2 nh 4 3h 2o 3.
ID: 1276550 Language: English School subject: Chemistry Grade/level: 8 Age: 12-17 Main content: Chemical Bonding Other contents: Add to my workbooks (10) Embed in my website or blog Add to Google Classroom
Practice Problems: Redox Reactions (Answer Key) Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 H: +1, O: -2, C: +4 b. N 2 N: 0 c. Zn(OH) 4 2-Zn: 2+, H: +1, O: -2 d. NO 2-N: +3, O: -2 e. LiH Li: +1, H: -1 f. Fe 3 O 4 Fe: +8/3, O: -2; Identify the species being oxidized and reduced in each of the ...
Redox practice worksheet Name: Date: 1. In which substance is the oxidation number of nitrogen zero? A. NH3 B. N2 C. NO2 D. N2O 2. What is the oxidation number of carbon in NaHCO3? A. +6 B. +2 C. 4 D. +4 3. In the reaction Al0 +Cr3+!Al3 +Cr0, the reducing agent is A. Al0 B. Cr3+ C. Al3+ D. Cr0 4. In the reaction 2K+Cl2!2KCl, the species ...
oxidation states, if the other atoms in the ion have known oxidation numbers. • Rule 4: The oxidation number of an alkali metal (IA family) in a compound is +1; the oxidation number of an alkaline earth metal (IIA family) in a compound is +2. • Rule 5: The oxidation number of oxygen in a compound is usually -2. If, however, the oxygen is
Give oxidation numbers for the underlined atoms in these molecules and ions. Oxidation number exercise answers page 57 oxidation number exercise do not hand in this work sheet. Notice the periodic trend among the main group. If the compound is an ionic compound the oxidation number for each element is the ion s. Oxidation numbers worksheet ...
Calculating Oxidation Numbers for Sulfur. We can begin by recalling that the charge on an ion corresponds to the sum of the oxidation numbers. (a) In S. 2-, the oxidation number of sulfur is - 2. (b) In SO. 3 2-, the polyatomic anion has a charge of 2 -. We assign oxygen an oxidation number of - 2 and write the equation (c) In SO. 4 2-
Oxidation Number Exercise page 51 Rule 2 Fluorine has an oxidation number of -1. Exercises - Give the oxidation number for the following atoms: NaF Na = IF 3 I = ClF 2-Cl = SF 4 S = PF 3 P = SF 6 2-S = PF 5 P = PF 6 3-P = W2F9 3-W = OF 2 O = NF 3 N = F2 F = Rule 3 The metals of group 1 (old CAS IA) have an oxidation number of +1
Microsoft Word - 14-04 Oxidation numbers worksheet.doc Author: Brent White Created Date: 7/15/2005 10:38:28 PM ...
20 Oxidation Number Worksheet with Answers. 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers worksheet answer key, assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, assigning oxidation number worksheet answers, oxidation number worksheet answers, via: scribd.com.
Rules for Assigning Oxidation Numbers The oxidation number of any uncombined element is 0 The oxidation number of a monatomic ion equals the charge on the ion. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. The oxidation number of fluorine in a compound is always -1.











![Worksheet - Assigning Oxidation Numbers - Key.doc [en5k2qr12eno]](https://idoc.pub/img/crop/300x300/en5k2qr12eno.jpg)


















0 Response to "42 oxidation number worksheet with answers"
Post a Comment