39 isotopes and average atomic mass worksheet
Atomic Mass Units (amu): roughly equal to the mass of a proton or neutron. **the mass of an atom is measured in amu's. 1 . amu = 1.66x10-24. g. Atomic Mass. The mass of an atom is determined using the number . of protons and neutrons in it. (electrons are ignored) Since the masses of atoms are so small in conventional mass units, Pre-AP Chemistry: Worksheet #3.3 Isotopes and Average Atomic Mass 1. Name two ways that isotopes of an element differ. 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
Worksheet Answer Key. 16 Best Images Of Atomic Structure Worksheet Answer Chart … , Atomic Structure Element Symbol Atomic Mass (Common Number Number Isotope) Hydrogen H 1 1 Helium He 2 4 Lithium Li Nitrogen N Oxygen O Silicon Si Krypton Kr Lead Pb Uranium U Plutonium Pu Isotopes Element 3 7 8 14 36 82 92 94 7 14 16 28 84 207 238 242 Number ...
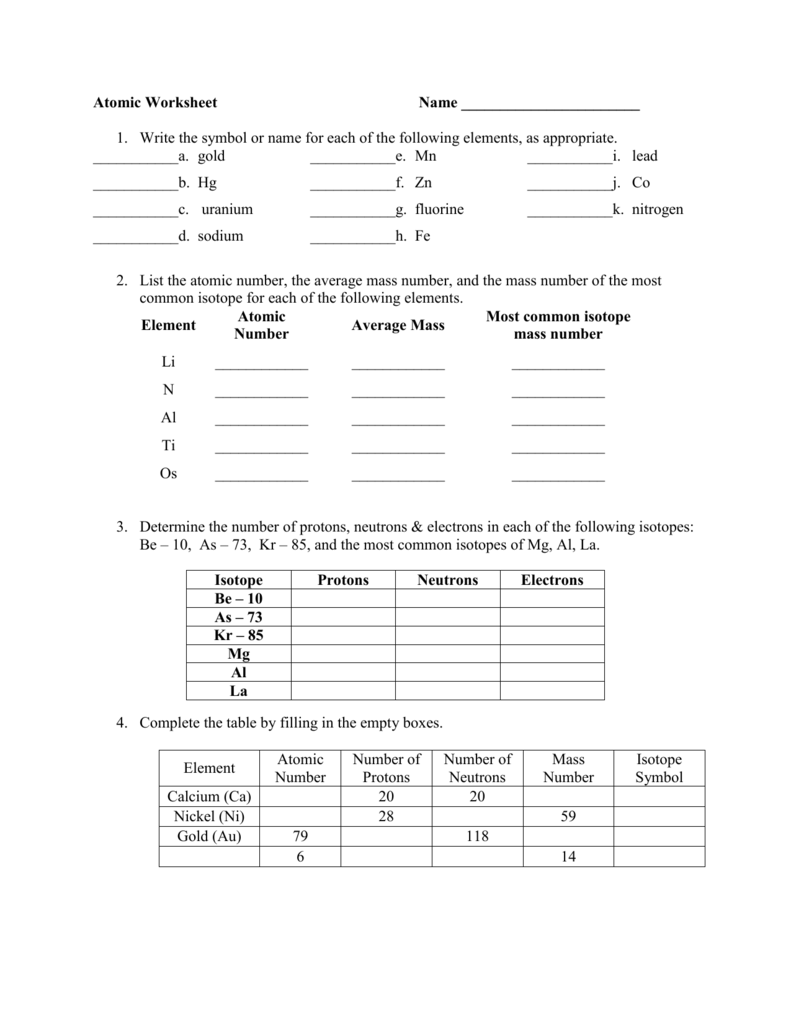
Isotopes and average atomic mass worksheet
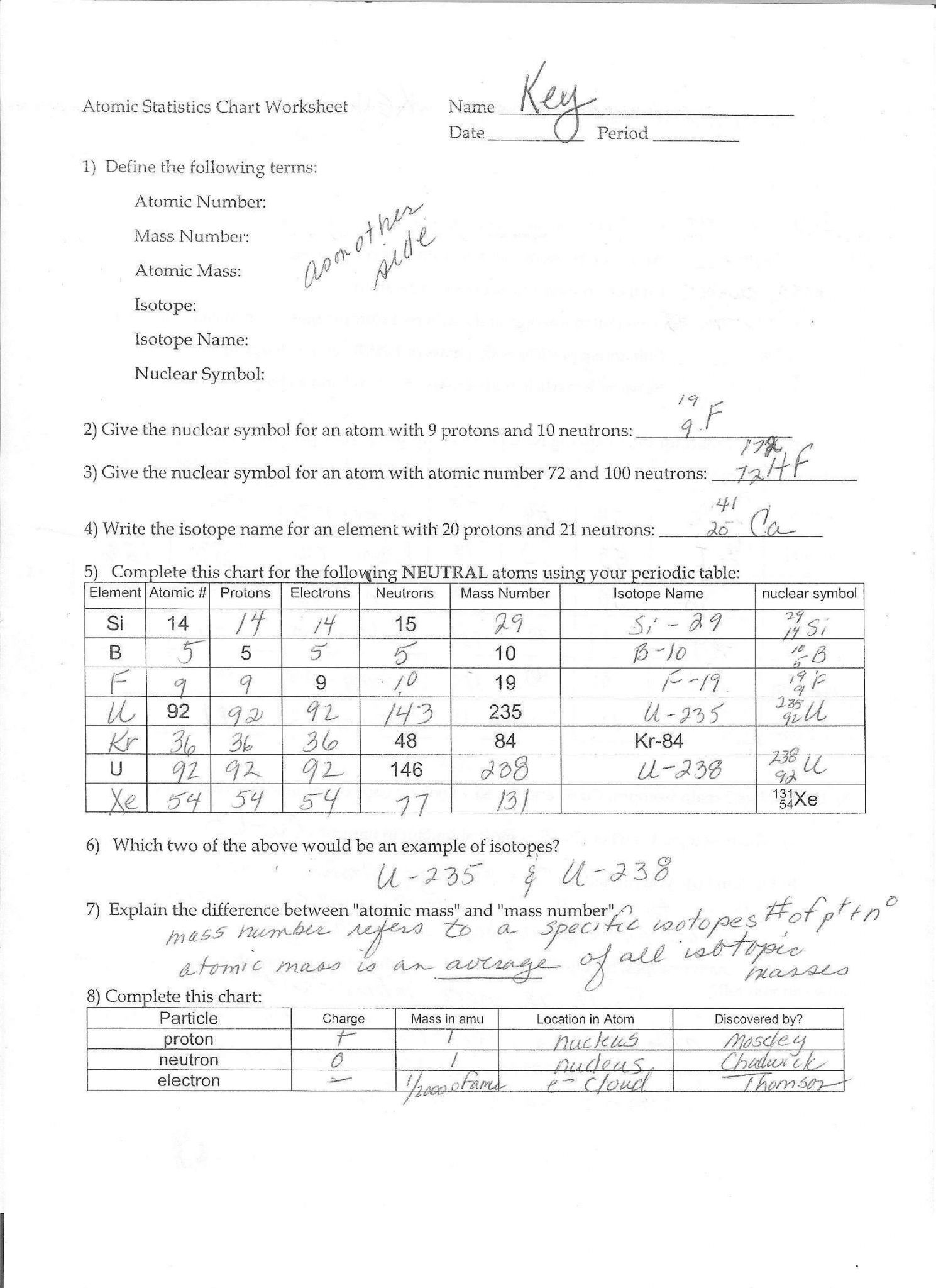
Isotope Isotope Notation Atomic # Protons Electrons Neutrons Nickel-58 15 15 53 74 36 48 34 45 Calcium-40 Chlorine-37 9. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Show all work. Mass of Isotope % abundance 36.96590 24.47 34.96885 75.53 isotope. Average atomic mass: a weighted average that uses the percent abundance and mass of all naturally occurring isotopes of the element. Carbon-12 is 98.2% abundant while Carbon-13 is 1.8% abundant. The atomic mass of carbon is 12.0111 amu and is closer to the more abundant isotope, carbon-12. Isotopes and Atomic Mass - PhET Interactive Simulations
Isotopes and average atomic mass worksheet. In a Physics class at school, what most people are taught is what is known as the Isotopes and Average atomic mass worksheet. The Isotopes are the number of protons and neutrons per unit volume of the earth's atmosphere. The Average atomic mass worksheet, on the other hand, is the number of atomic masses per unit volume of the earth's atmosphere. a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu. Atomic masses of isotopes vary slightly and only carbon-12 has an atomic mass that is an integer since the amu is defined relative to the mass of carbon-12. Science Inquiry A great activity for the class is to calculate average atomic mass of an element called "vegium", which consists of three isotopes - beans, peas, and corn.
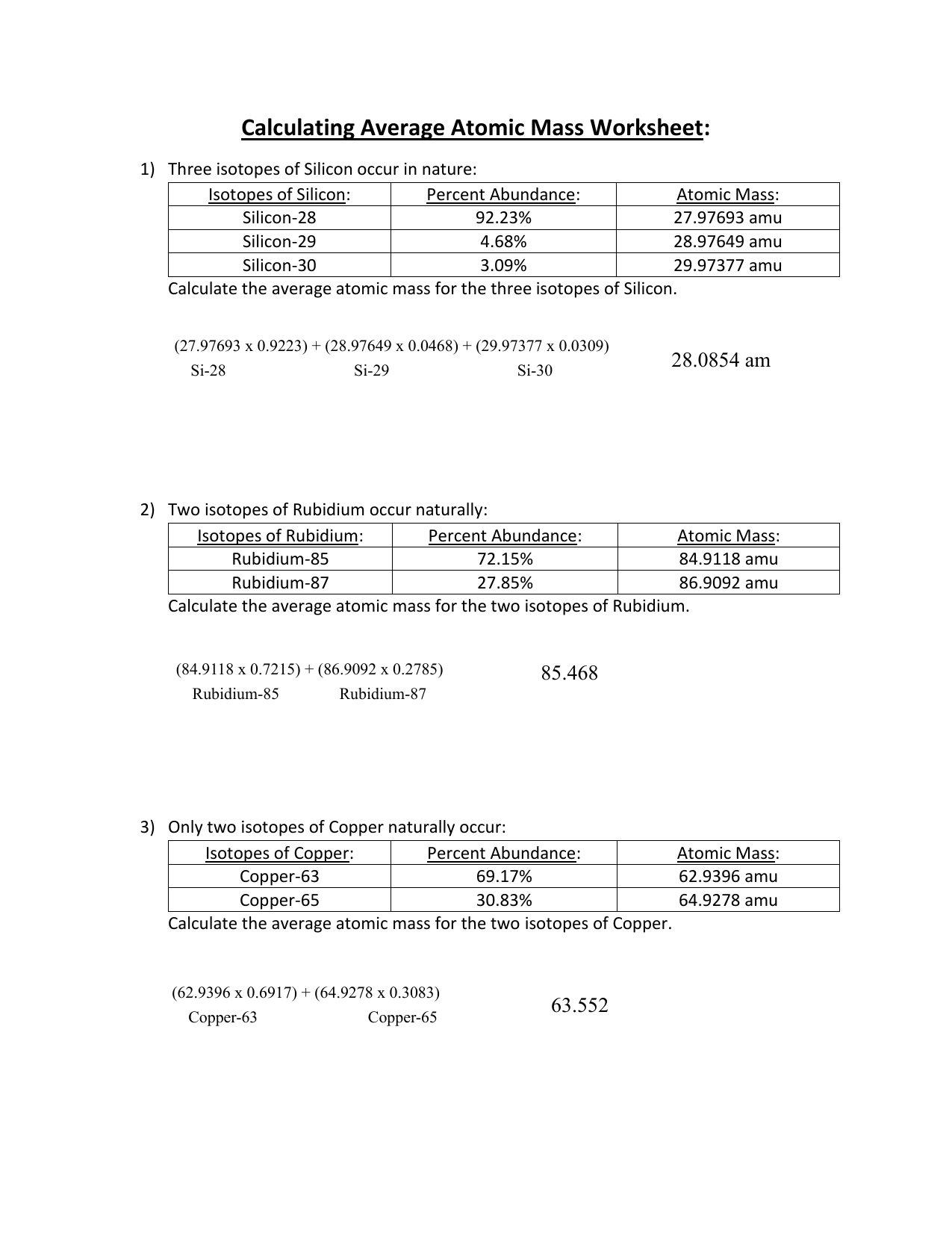
About This Quiz & Worksheet Isotopes and average atomic mass, as concepts, allow for the specific discussion of elements and their atoms, and this quiz/worksheet combo will help you test your... name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. Pre-AP Chemistry: Worksheet #3.3 Isotopes and Average Atomic Mass 1. Name two ways that isotopes of an element differ. Mass Number, Atomic Mass, Neutrons 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? Atomic Mass of each isotope and % abundance of each isotope 3. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
Calculate the average atomic mass of this Potassium sample. The three Potassium isotopes have atomic masses and percent compositions of 39 amu (64.8%), 40 amu (18.6%), and 41 amu (16.6%). Complete the following chart: Element Symbol Atomic Number Number of electrons Number of Neutrons Mass number Isotope Notation Atomic Mass Helium. 2 2 Ti 22 ... T 17- 3 I sðf 8 197 198 19 q. Isotopes and Atomic mass worksheet The atomic mass of an element as stated in the periodic table is the weighted average of all of the known isotopes of that element. Calculate the actual atomic mass of 65Cu. The average atomic mass between these two isotopes is 63546 amu. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 32, 0.76% has a mass of 33 and 4.22% have a mass of 34. 3. The four isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus. 100 = 10.811 (note that this is the value of atomic mass given on the periodic table) *amu is the atomic mass unit (u, μ or amu), which is defined as 1/12th the mass of a carbon-12 atom. This value is arbitrary and simply provides a reference point for measuring relative atomic masses. Stable vs. Unstable Isotopes
7. Boron exists in two isotopes, boron-10 and boron-11. Based on the atomic mass, which isotope should be more abundant? Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of ...
In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6.
Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
6. Weighted average of naturally occurring isotopes atomic mass 7. Total number of protons plus neutrons mass number 8. All electrons are in the lowest energy levels ground state 9. 1/12 the mass of a carbon-12 atom atomic mass unit (u) 10. How to solve for the number of neutrons mass number - atomic number 11.
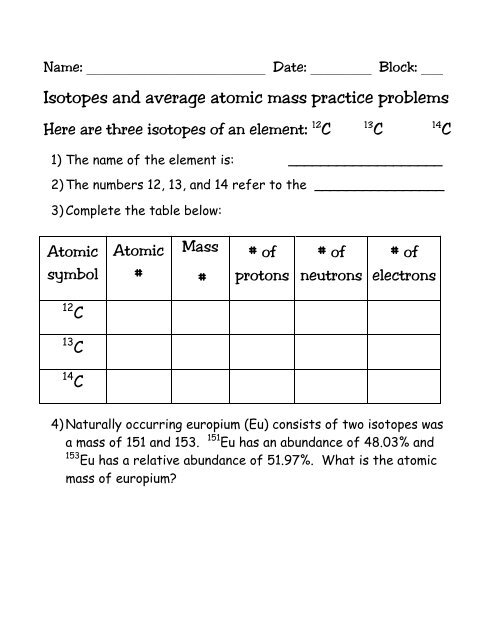
U1L4: Average Atomic Mass and Isotopes Worksheet 1. The average atomic mass of carbon is 12.01 u. Explain why no single atom of carbon has an atomic mass of 12.01 u. Provide the 2 types of isotopes of carbon. It is because the average atomic mass represents the average of all isotopes.
ISOTOPES AND AVERAGE ATOMIC MASS Name Elements come in a variety of isotopes, meaning they are made up of atoms with the same atomic number but different atomic masses. These atoms differ in the number of neutrons. The average atomic mass is the weighted average of all the isotopes of an element.
Worksheet - Isotopes and Average Atomic masses Name_ Period. Date 1. Four isotopes of lead include lead-204, lead-206, lead-207, and lead-208. The average atomic mass of a lead atom is 207.2 amu. Which isotope of lead is likely to be the most abundant? 2. What do all isotopes of an element have in common? 3.
Nickel-58 15 15 53 74 36 48 34 45 Calcium-40 Chlorine-37 Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Show all work. Mass of Isotope % abundance 36.96590 24.47 34.96885 75.53 Atoms and Isotopes Worksheet. Fill in the table with the correct information
To calculate average atomic mass of an element: Average atomic mass = (fractional abundance of isotope 1)(atomic mass of isotope 1) + (fractional abundance of isotope 2)(atomic mass of isotope 2) + . . . . . . Practice Problems 1. Chlorine has two isotopes. Chlorine-35 has an actual mass of 34.9689 u and chlorine-37 has a mass of 36.9659 u.
Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu.
This quiz and worksheet pairing will help check your knowledge of the structure and functionality for radioactive isotopes. The average atomic mass is the weighted average of all the isotopes of an element. The average atomic mass between these two isotopes is 63546 amu. A sample of cesium is 75 133Cs 20 132Cs and 5 134Cs. What is an isotope.
Isotopes and Atomic Mass - PhET Interactive Simulations
isotope. Average atomic mass: a weighted average that uses the percent abundance and mass of all naturally occurring isotopes of the element. Carbon-12 is 98.2% abundant while Carbon-13 is 1.8% abundant. The atomic mass of carbon is 12.0111 amu and is closer to the more abundant isotope, carbon-12.
Isotope Isotope Notation Atomic # Protons Electrons Neutrons Nickel-58 15 15 53 74 36 48 34 45 Calcium-40 Chlorine-37 9. Calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Show all work. Mass of Isotope % abundance 36.96590 24.47 34.96885 75.53


























0 Response to "39 isotopes and average atomic mass worksheet"
Post a Comment