39 wavelength frequency and energy worksheet answers
Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) Wavelength Frequency And Energy Worksheet Answers. These worksheets explain how to use frequency tables to tally the frequency of ... Documents Similar To energy work power worksheet answer key. ... to calculate the energy intensity for every wavelength and every temperature, respectively..
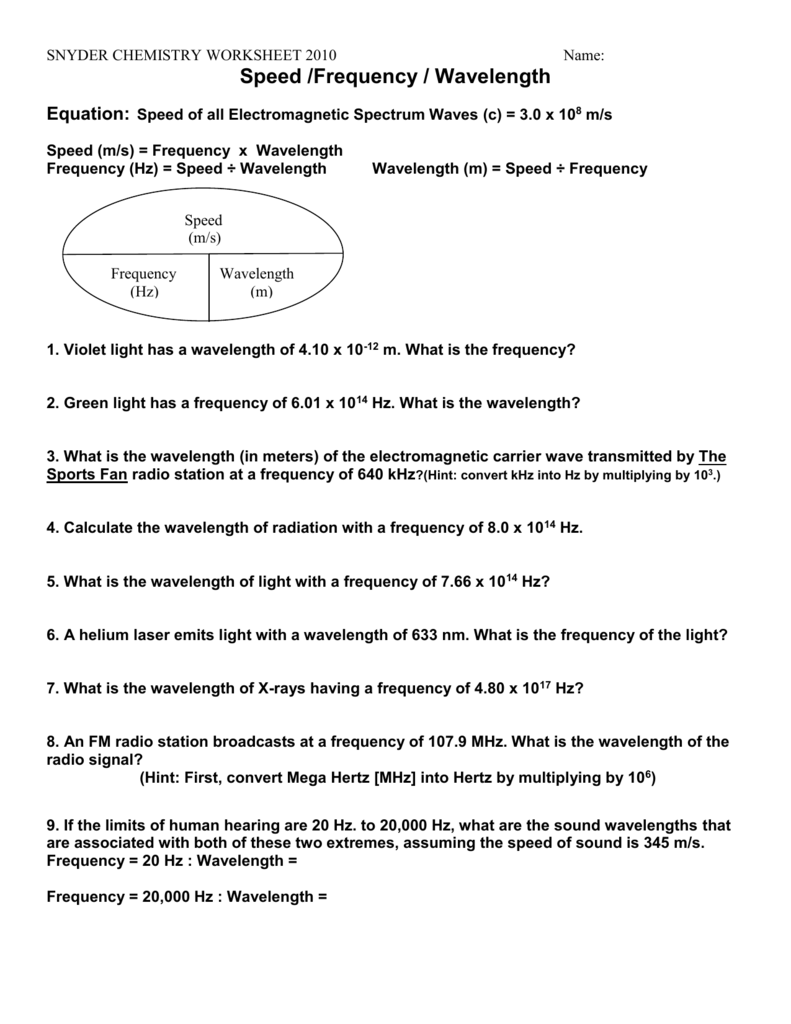
Chemistry Worksheet and Answers Wavelength, frequency, & energy of electromagnetic waves. C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) 1. What is the wavelength of a wave having a frequency of 3.76 x 1014 s-1? 2.

Wavelength frequency and energy worksheet answers
Calculate the energy of a photon of radiation with a wavelength of 6.4 x 10-7 m. 1st, need frequency f= c/w= 3.00x108/6.4*10-7 = .468 x 10*(8--7) = .468x1015 = 4.68x1014 Hz 2nd, use 4.68x1014 Hz to calculate energy Energyphoton = 4.68x1014 x 6.626 x 10-34 Joules 44.26168 x 10(14+-34) Joules = 44.26168 x 10-20 Joules = 4.426168 x 10-19 Joules Energy / Frequency / Wavelength Energy (J) = h x ν h (Planck’s Constant) = 6.626 x 10-34 J . s (Joules) 10. Calculate the energy of a photon of radiation with a frequency of 8.5 x 1014 Hz. -5.63 × 10 19 J 11. Calculate the energy of a gamma ray photon whose frequency is 5.02 x 1020 Hz? 3.33 -× 10 13 J 12. Calculate the energy of a photon ... nu (or new) is the frequency of a wave, and c is the speed of light (a constant equal to. 3.0 x 10 m/s.) Equantum = hy where Equantum is the energy of a ...3 pages
Wavelength frequency and energy worksheet answers. Energy / Frequency / Wavelength Energy (J) = h x Frequency h (Planck’s Constant) = 6.626 x 10-34 J .s Energy = h x (c ÷ wavelength) 9. Calculate the energy of a photon of radiation with a frequency of 8.5 x 1014 Hz. 10. Calculate the energy of a gamma ray photon whose frequency is 5.02 x 1020 Hz? 11. Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY. Show ALL equations, work, units, and significant figures in ...4 pages Answer key. Chemistry Worksheet - Wavelength, frequency, & energy of electromagnetic waves. Show ALL equations, work, units, and significant figures in ... E = h! and c =!!" E = energy (J) = wavelength (m) ! = frequency (Hz or s-1) h = Planck’s constant, 6.626x10-34J∙s c = the speed of light in a vacuum, 3.00 × 108m∙s-1 During the course of this unit, you should become very comfortable with the process of solving problems like the following.
What is the wavelength of a wave having a frequency of 3.76 x 1014 s-1? 2. ... Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the ... Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves 11. Calculate the energy of a photon of radiation with a wavelength of 6.4 x 10-7m. E=16.626*10-34) ... Calculate the wavelength of radiation with a frequency of 8.0 x 1014 Hz. Calculating Energy and Frequency (f) Calculate the energy of a photon of radiation with a frequency of 8.5 x 1014 Hz. Calculate the energy of a gamma ray photon whose frequency is 5.02 x 1020 Hz? Calculate the energy of a photon of radiation with a wavelength of 6.4 x 10-7 m. name: suggested answers date: _____ wavelength, frequency, and energy – practice problems 1. Calculate the frequency of green light, which has a wavelength of 4.90 x 10-7 m. 2. Calculate the frequency and energy of an X-ray that has a wavelength of 1.15 x 10-10 m. 3. Calculate the wavelength of an electromagnetic wave with a frequency of 7.8 ...
nu (or new) is the frequency of a wave, and c is the speed of light (a constant equal to. 3.0 x 10 m/s.) Equantum = hy where Equantum is the energy of a ...3 pages Energy / Frequency / Wavelength Energy (J) = h x ν h (Planck’s Constant) = 6.626 x 10-34 J . s (Joules) 10. Calculate the energy of a photon of radiation with a frequency of 8.5 x 1014 Hz. -5.63 × 10 19 J 11. Calculate the energy of a gamma ray photon whose frequency is 5.02 x 1020 Hz? 3.33 -× 10 13 J 12. Calculate the energy of a photon ... Calculate the energy of a photon of radiation with a wavelength of 6.4 x 10-7 m. 1st, need frequency f= c/w= 3.00x108/6.4*10-7 = .468 x 10*(8--7) = .468x1015 = 4.68x1014 Hz 2nd, use 4.68x1014 Hz to calculate energy Energyphoton = 4.68x1014 x 6.626 x 10-34 Joules 44.26168 x 10(14+-34) Joules = 44.26168 x 10-20 Joules = 4.426168 x 10-19 Joules

























0 Response to "39 wavelength frequency and energy worksheet answers"
Post a Comment