T2 = 894 K-1621°C]. Page 3. Combined Gas Law Problems: atm = 1600 mm. Pivi. Ti. PV. T2. Tatma 76. 1 . VU VIIHOT102D. 6 : LUI. I. 760.3 pages Give the answer in Kelvin and °C? 4) A 113L sample of Helium at 27°C is cooled to -78°C. Calculate the new volume of the Helium ...2 pages
Use the combined gas law to solve the following problems: 1) if ! initially have a gas at a pressure of 12'atm, a volume of 23 liters, and a.2 pages

Charles law problems worksheet answers
In all the problems below, the pressure and the amount of gas are held constant. ... x = 54.5 mL <--- that's the ending volume, which is NOT the answer. Key. Charles' Law states that the volume of a gas varies directly with the Kelvin temperature ... Solve the following problems assuming a constant pressure.3 pages Combined Gas Law Problems: 1. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °C and the pressure is 740.0 mm of mercury ...4 pages
Charles law problems worksheet answers. 16 Nov 2019 — Problems. Solve the following problems. Problem 1. Hydrogen gas contracts at constant pressure from 1.00 L to 0.95 L. The initial temperature is ... Name: KEY. Name: Gas Laws Worksheet #1 - Boyle's, Charles', Gay-Lussac's, and Combined Gas Law. Solve all problems - you must show your work (including ...22 pages Charles's Law - volume and temperature changes at constant pressure 4. ... Boyle's and Charles's Laws Practice Problems Answer Key 1. 55.3 mL 2. 3.18 atm 3. Combined Gas Law Problems: 1. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °C and the pressure is 740.0 mm of mercury ...4 pages
Key. Charles' Law states that the volume of a gas varies directly with the Kelvin temperature ... Solve the following problems assuming a constant pressure.3 pages In all the problems below, the pressure and the amount of gas are held constant. ... x = 54.5 mL <--- that's the ending volume, which is NOT the answer.

13 Best Images of Pressure Problems Worksheet Answer Key ...

Mute swan swimming on green lake.

Charles Law Practice Problems Worksheet Answer Key ...
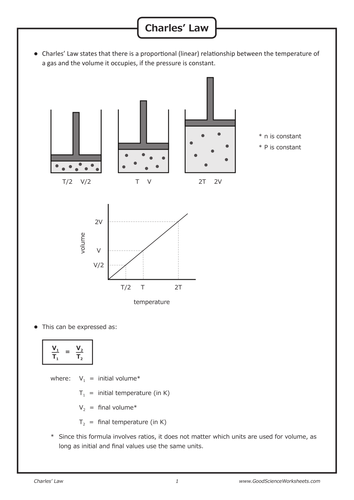
Gas Laws - Charles' Law by GoodScienceWorksheets ...

Boyles Law Worksheet Answer Key - Worksheet List

Charles Law Worksheet Answers - Nidecmege

Combined Gas Law Problems Worksheet

Boyles Law Worksheet - worksheet

32 Charles Law Problems Worksheet Answers - Notutahituq ...
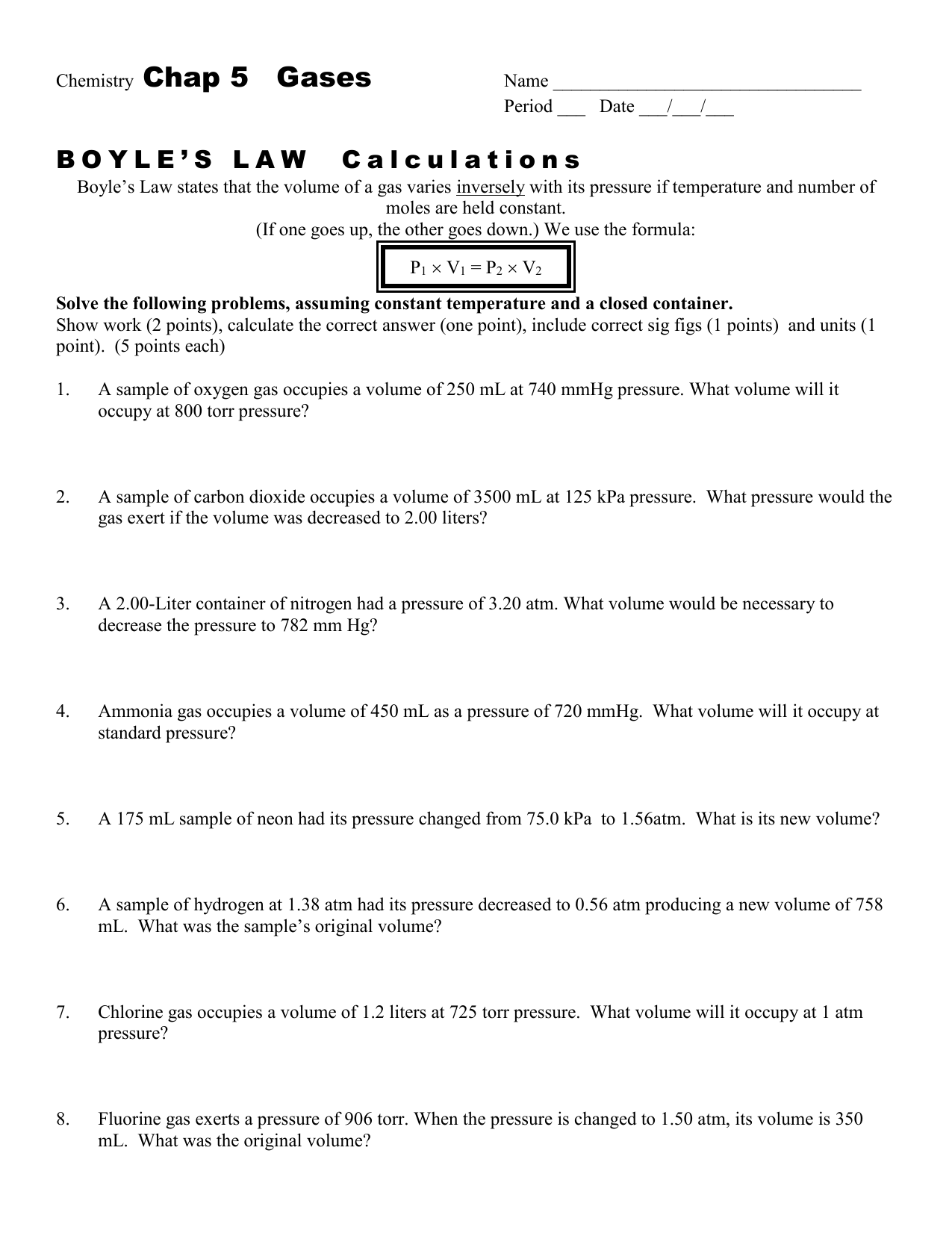
Boyle S Law Worksheet Answers Chapter 12 - worksheet

26 Charles Law Problems Worksheet Answers - Worksheet ...

Charles Law Worksheet 1 Answer Key - Instantworksheet

Charles Law Practice Problems Worksheet Answer Key ...

Boyles Law Worksheet - worksheet

Charles Law Worksheet | Homeschooldressage.com

6 Mole Calculation Practice Worksheet Answers | FabTemplatez

Yellow and White Flowers.

Assignments/Labs - ERHS Chemistry with Mr. Stagg

Combined Gas Law Worksheet Answers

Charles Law Problems Worksheet Answers - worksheet

Charles Law Worksheet - Thekidsworksheet

Ideal Gas Law Worksheet Answer Key - Askworksheet

Solving Combined Gas Law Problems - Charles' Law, Boyle's ...

Charles Law Problems Worksheet Answers - Worksheet List

Charles Law Worksheet Answers - Nidecmege

BOYLES AND CHARLES LAW WORKSHEET WITH ANSWERS | Teaching ...

Boyle's Law and Charles Law Worksheet Answer Key ...

Ideal Gas Law Practice Worksheet - kidsworksheetfun

Boyle's Law and Charles Law Worksheet Answer Key ...

Wisps of green pampas grass catching the sunlight.

Ideal Gas Law Worksheet Answer Key - Askworksheet

Charles Law Worksheet.pdf

Purple flowers in flower garden.

Boyles And Charles Law Worksheet Answers Gas Law Worksheet ...

Gas Laws Worksheet III Answer Key 11-12 | Gases | Mole (Unit)

Gas Laws Worksheet Answers | Homeschooldressage.com

31 Charles Law Worksheet Answers - Worksheet Information

11 Best Images of Ohms Law Worksheet Answers - Ohms Law ...

Boyle S Law Worksheet Answers Chapter 12 - worksheet

Charles Law Problems Worksheet Answers - worksheet

Ideal Gas Law Problems Worksheet | Homeschooldressage.com


































0 Response to "41 charles law problems worksheet answers"
Post a Comment