43 oxidation numbers worksheet answers
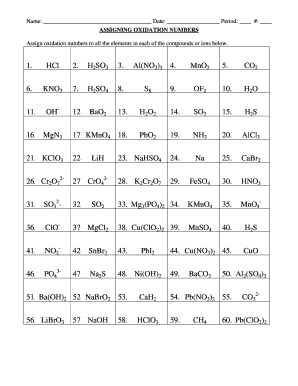
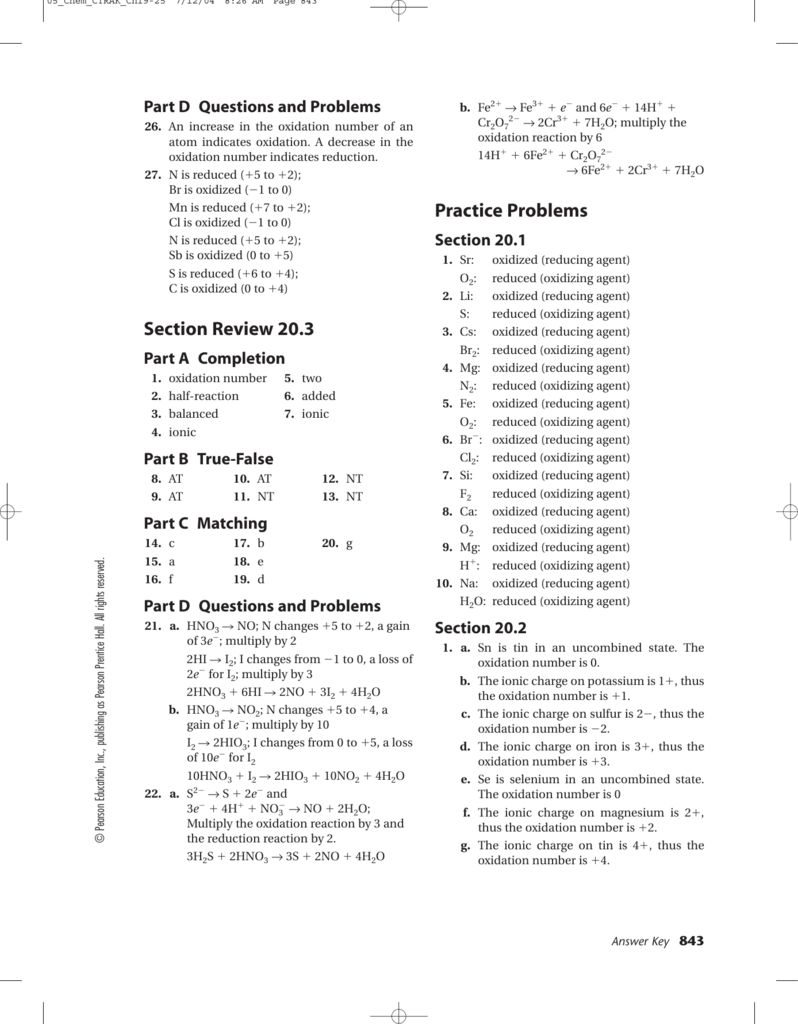
Jan 18, 2022 · The oxidation number of an element in a monatomic ion equals the charge of the ion. 4th Grade Math Worksheets. 00×102 mL at a temperature of 20. 2 days agoThe answer key helps the candidates to check their numbers of correct answers and wrong answers. worksheet oxidation numbers answer key. Worksheet Oxidation Numbers Written By admin Monday ... Oxidation Number Exercise - answers Page 57 Oxidation Number Exercise Do not hand in this work sheet. When you are ready, you will be given an examination over this material. Complete the examination by yourself and hand it in to receive credit. Purpose: This exercise is designed to teach the student how to assign oxidation numbers.
Apr 07, 2015 · Sc, Y and Al have an oxidation number of +3. Exercises - Give the oxidation number for the following atoms: Na 2O Na = Na 2O2 O = KO 2 O = NaOH Na = ScH 3 H = LiH H = CaC 2 C = CaMgO 2 O = MgH 2 H = MgF 2 Mg = RbO 2 O = MgSF 6 S = NaPF 6 P = LiBF 4 B = Rule 4 Hydrogen has an oxidation number of +1 when combined with elements on

Oxidation numbers worksheet answers
20 Oxidation Number Worksheet with Answers. 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers worksheet answer key, assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, assigning oxidation number worksheet answers, oxidation number worksheet answers, via: scribd.com. The oxidation number of any uncombined element is 0 The oxidation number of a monatomic ion equals the charge on the ion. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. The oxidation number of fluorine in a compound is always -1. Oxygen has an oxidation number of -2 unless it is … 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, rules for assigning oxidation numbers worksheet answers, chapter 7 charting oxidation number worksheet answers, assigning oxidation number worksheet answers, , image source: scribd.com.
Oxidation numbers worksheet answers. Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ... Oxidation numbers worksheet answers. Cl 2 cl 16. The oxidation number of barium identifying charges in given substances identifying oxidation numbers in given compounds skills practiced. Sio 2 si o 3. Microsoft word 14 04 oxidation numbers worksheet doc author. Oxidation number exercise answers page 57 oxidation number exercise do not hand in this work sheet. Jan 06, 2022 · Assigning oxidation numbers worksheet answer key. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. Al s cl2 g o3 g the total oxidation number of a neutral compounds 0 co2 h2o the oxidation number of a monatomic ion is equal to its charge. Sep 13, 2021 · Oxidation numbers worksheet answers. The oxidation number of fluorine in a compound is always 1. The oxidation number of fluorine in a …
Chem 12 Practice Worksheet - Answer Key Key page 1 Redox #1 (KEY) 1. Explain the meaning of each of the following terms: a) oxidation a half-reaction that involves the loss of electron(s) b) reduction a half-reaction that involves the gain of electron(s) c) reducing agent a species that causes another to be reduced; it itself is oxidized d) oxidizing agent a species that causes another to be ... Rules for Assigning Oxidation Numbers 1. The oxidation number of any uncombined element is 0. 2. The oxidation number of a monatomic ion equals the charge on the ion. 3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. 4. The oxidation number of fluorine in a compound is always -1. 5. Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 ... For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of oxygen in H2O2? -1 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, rules for assigning oxidation numbers worksheet answers, chapter 7 charting oxidation number worksheet answers, assigning oxidation number worksheet answers, , image source: scribd.com.
The oxidation number of any uncombined element is 0 The oxidation number of a monatomic ion equals the charge on the ion. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. The oxidation number of fluorine in a compound is always -1. Oxygen has an oxidation number of -2 unless it is … 20 Oxidation Number Worksheet with Answers. 07 Finding Oxidation Numbers Worksheetc Ion assigning oxidation numbers worksheet answer key, assigning oxidation numbers practice worksheet answers, oxidation numbers worksheet answers rules used, assigning oxidation number worksheet answers, oxidation number worksheet answers, via: scribd.com.





























0 Response to "43 oxidation numbers worksheet answers"
Post a Comment