45 11.1 describing chemical reactions worksheet answers
PDF Chapter 11: Stoichiometry - Mr. Miller's Classes Figure 11.1is as follows. 4Fe(s) +3 O 2 (g) →2F e 2 O 3 (s) You can interpret this equation in terms of representative particles by saying that four atoms of iron react with three molecules of oxygen to produce two formula units of iron(III) oxide. Chapter 11 Cooking food always involves a chemical reaction. •The recipe tells you which ingredients to mix together and how much of each to use. •Chemical reactions take place when the ingredients or reactants are mixed together and heated in the oven. •The product, in this case, is a batch of muffins. 11.1 Chemical Reactions >
Chapter 11 - Chemical Reactions - 11.1 Describing Chemical Reactions ... Chapter 11 - Chemical Reactions - 11.1 Describing Chemical Reactions - 11.1 Lesson Check - Page 354: 7 Answer Write the chemical formulas of the reactants on the left of the yield sign and the chemical formulas of the products on the right of the yield sign. Work Step by Step

11.1 describing chemical reactions worksheet answers
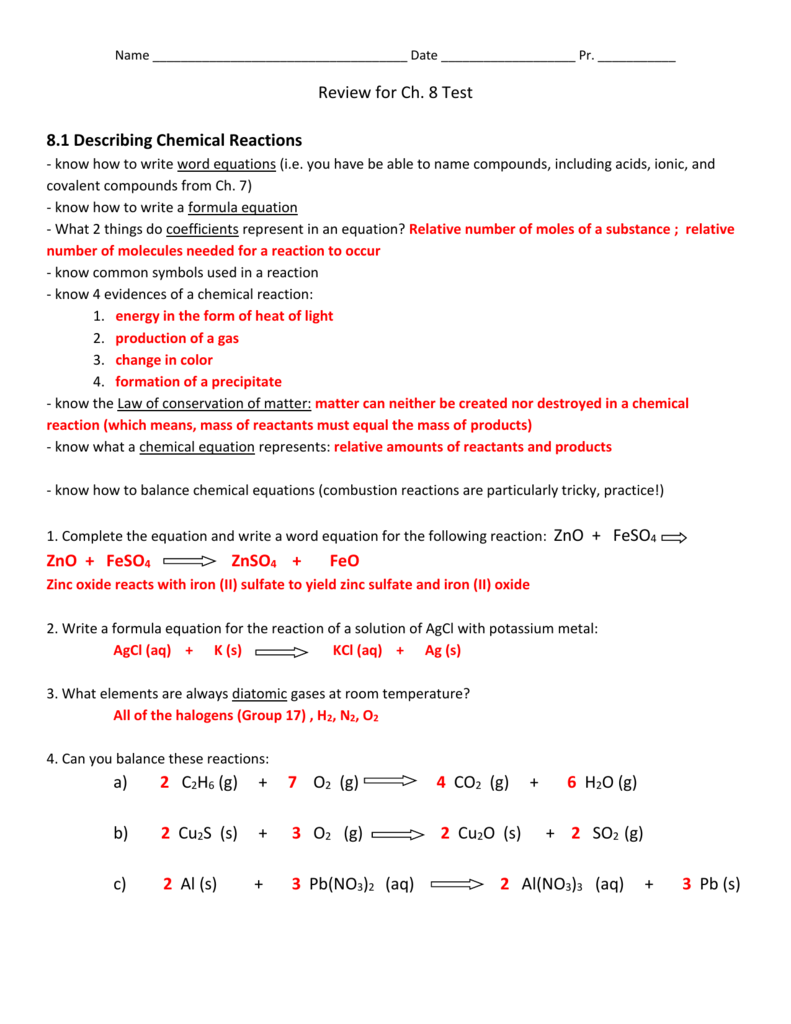
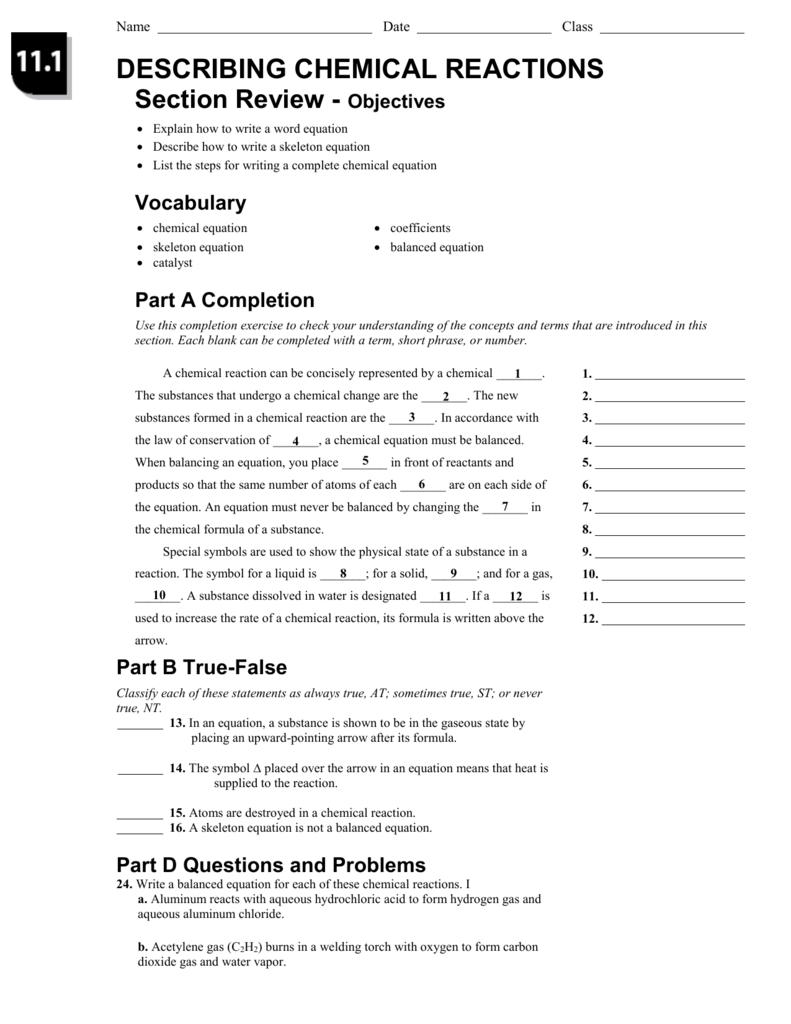
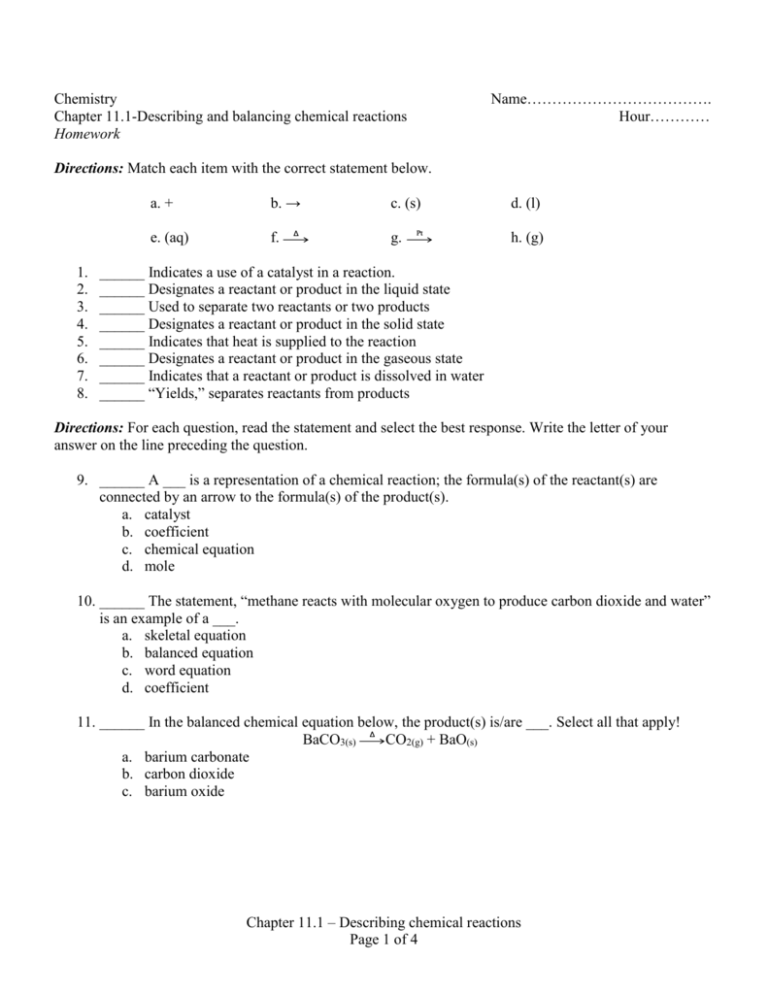
Access Free 111 Describing Chemical Reactions Answers Chapter 11: chemical reactions. 1. Deter- mine the correct formulas for all the reac- tants and products 2. Write the skeleton equation 3. Determine the number of atoms of each element in the reactants and products 4. Balance the elements one at a time by using coefficients 5. Check each atom or poly atomic ion to make sure its equal 6. 11.1 Describing Chemical Reactions Flashcards | Quizlet 11.1 Describing Chemical Reactions Flashcards | Quizlet Science Engineering Chemical Engineering 11.1 Describing Chemical Reactions 4.7 6 Reviews STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Equation Click card to see definition 👆 A chemical reaction can be concisely represented by a chemical ____ Click again to see term 👆 1/23 CHE 105/110 - Introduction to Chemistry - Textbook Historically, the first chemical definition of an acid and a base was put forward by Svante Arrhenius, a Swedish chemist, in 1884. An Arrhenius acid is a compound that increases the H + ion concentration in aqueous solution. The H + ion is just a bare proton, and it is rather clear that bare protons are not floating around in an aqueous solution. Instead, chemistry has defined thehydronium ion ...
11.1 describing chemical reactions worksheet answers. CK-12 Chemistry Concepts - Intermediate Answer Key Chapter 11: Chemical ... Answers 1. When two or more substances combine to form a new substance. 2. Mg(OH) 2 3. No, two substances are formed. In a combination reaction, only one substance is formed. 11.5 Decomposition Reactions Practice Questions Write the reactions (including names and balanced equations) as requested on the following web site: PDF Practice Problems - Chemistry Department - chem.usu.edu The reaction for a weak acid can be written as HA + 1-120 + A-. The acid dissociation constant expression is written as [HA] BH+ + OH , and the base dissociation constant expression is For a weak base the equation is B + H20 written as [B] Practice Problems 25. Answer true or false for each of the following: A strong acid 11.1 describing chemical reactions Flashcards | Quizlet a chemical equation uses chemical formulas instead of words to describe a chemical reaction. A chemical reaction that shows only the formulas but not the relative amounts of the reactants and products is a. skeleton equation. reactants. Li and Br2. ... Verified answer. ENGINEERING. For each of the following substances, compute the final ... Read Online Chapter 11 Chemical Reactions D Reading Answer Key SECTION 11.1 DESCRIBING CHEMICAL REACTIONS (pages 321-329) reactions, we can predict the products of reactions using these steps: 1. Determine what kind of reaction the reactants will most likely form 2. Look at the solubility rules to determine if a solid formed 3. Balance the chemical reaction
CK-12 Chemistry Concepts - Intermediate Answer Key Chapter 11: Chemical ... Answers 1. When two or more substances combine to form a new substance. 2. Mg(OH) 2 3. No, two substances ere formed. In a combination reaction, only one substance is formed. 11.5 Decomposition Reactions Practice Questions Write the reactions (including names and balanced equations) as requested on the following web site: PDF from Organic Chemistry 1. Organic Molecules and Chemical Bonding 2. Alkanes and Cycloalkanes 3. Haloalkanes, Alcohols, Ethers, and Amines 4. Stereochemistry 5. Organic Spectrometry II. Reactions, Mechanisms, Multiple Bonds 6. Organic Reactions *(Not yet Posted) 7. Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution 8. Alkenes and Alkynes 9. Get Free 111 Describing Chemical Reactions Answers 111 Describing Chemical Reactions Worksheet Answers if a __ is used to increase the rate of a chemical reaction, its formula is written above the arrow ST in an equation, a subs-tance is shown to be in the gaseous state by placing an upward-pointing arrow after its formula 11.1 Describing Chemical Reactions Flashcards | Quizlet 11 1 Describing ... PDF Chapter 11 Gases 11.1 Gases and Their Properties If you want to understand how gases behave—such as why fresh air rushes into your lungs when certain chest muscles contract or how gases in a car's engine move the pistons and power the car—you need a clear mental image of the model chemists use to explain the properties of gases and the relationships between them.
11.1_ Describing Chemical Reactions.pdf - Number your answers 1-10. If ... Number your answers 1-10. If your answers need a superscript or subscript, use the superscript (T x) and/or subscript (T x) buttons in the text editor or word processor. Answer the following questions. (8 points, 2 points each) What is a chemical equation? A chemical reaction is when two substances are put together and a new substance is formed during the process. Chapter 11 - Chemical Reactions - 11.1 Describing Chemical Reactions ... Chapter 11 - Chemical Reactions - 11.1 Describing Chemical Reactions - 11.1 Lesson Check - Page 354: 9 Answer 2SO2 + O2 -> 2SO3 Work Step by Step Balance the skeleton coefficient using coefficients so there is the same number of atoms in the reactants and products. Update this answer! Unit 12: Answer keys, Reviews, + Handouts - PiersonHChem - Google 1.1: Describing matter. 1.2: What is Chemistry? 1.3: States of Matter. ... Unit 9: Chemical Reactions. 9.1: Writing chemical reactions. 9.2 Balancing Reactions. 9.3: Reaction Types. 9.4: Net Ionic Equations. ... Answer Keys; Selection File type icon File name Description Size Revision Time Read Book Prentice Hall Chemistry Chapter 11 Answer Key BFS0IJ Prentice Hall Chemistry Chapter 11 Answer Key 1 ... Describing Chemical Reactions FSc Chemistry Book1, CH 11, LEC 2: Rate of Reaction 12th Class Chemistry Ch 11 Live Lecture - FSc Chemistry book 2 ch 11 Alcohols,Phenols and Ethers 10th Class Chemistry, ch 11, Introduction Organic Compound - Ma-
PDF Chapter 11 Acids and Bases Practice Problems Section 11.1 Acids and ... 25. Answer true or false for each of the following: A strong acid… a. is completely dissociated in aqueous solution b. has a small value of K a c. has a strong conjugate base d. has a weak conjugate base e. is slightly dissociated in aqueous solution 26. Answer true or false for each of the following: A weak acid…
PDF The Mole - Nectur 11. 1.20 mol Cu !"7.22 ! 1023 Cu atoms 12. 9.25 ! 1022 ... Describe how to find the percent composition of a compound if you know the mass of a ... In your textbook, read about empirical and molecular formulas. Circle the letter of the choice that best answers the question. 3. Which information about a compound can you use to begin to determine ...
identifying chemical reactions worksheet reactions describing chemical worksheet answers section problems practice dokumen source tips. Section 11.1 describing chemical reactions worksheet answers. Types worksheet identifying reactions reaction chemical answers organic lewis compounds dot balancing equations chemistry answer key structures worksheeto worksheets via.
11.3 Reactions in Aqueous Solution - Quia (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq) •When sodium chloride dissolves in water, it separates into sodium ions (Na+(aq)) and chloride ions (Cl-(aq)). •When dissolved in water, silver nitrate dissociates into silver ions (Ag+(aq)). 11.3 Reactions in Aqueous Solution 9 > Net Ionic Equations
Honors Chemistry - PLHS Science Lewis Dot Worksheet Chap 8 Review Answers. Chapter 9 Chemical Names & Formulas. Power Points: 9.1 Naming Ions ... 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in an Aqueous Solution Chapter 11 Review Key. Chapter 12 - Stoichiometry.
PDF 11 - National Institute of Open Schooling Endothermic reactions are those reactions which proceed with the absorption of heat from the surroundings. 11.3 Thermochemical Equations You are familiar with equations for chemical reactions. Now we shall write the chemical equations which will specify heat energy changes and states of the reactants and products.
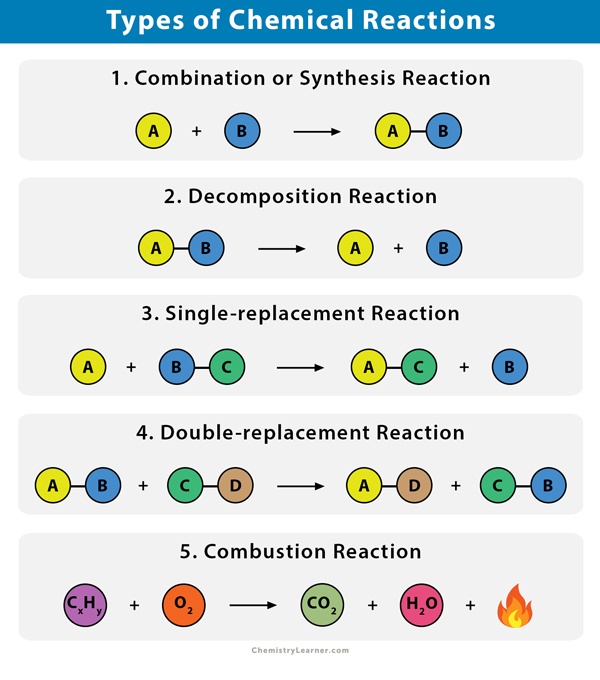
11.2 Types of Chemical Reactions> - Quia 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution ... 11.2 Types of Chemical Reactions> 29 Whether one metal will displace another metal from a compound depends upon the relative reactivities of the two metals.
11.1: Radioactivity - Chemistry LibreTexts Answer: Alpha, beta, and gamma emissions have different abilities to penetrate matter. The relatively large alpha particle is easily stopped by matter (although it may impart a significant amount of energy to the matter it contacts). Beta particles penetrate slightly into matter, perhaps a few centimeters at most.
UNIT 1- Chemical Reactions - Ms. Gauthier - Read p. 225 - 226; Lesson 6.1- Describing Chemical Reactions: Mon, Oct. 1: LESSON: 6.1, 6.3 & 6.4- Describing & Balancing Chemical Reactions *Student Copy - Concept Check: Counting Atoms Worksheet; (*ANSWERS) ... SIDE 2: "Acids and Bases Worksheet" *ANSWERS - Complete Pre-Lab for tomorrow
DOC Acid and Base Worksheet - Answers 4) Write an equation for each of the following reactions and indicate what each. reaction produces: (For each of the following oxides, indicate if it is an acid or basic anhydride). a. Carbon dioxide and water. CO2 + H2O ( H2CO3. Acid anhydride. b. Sodium oxide and water. Na2O + H2O ( 2NaOH(aq) Basic anhydride
CHE 105/110 - Introduction to Chemistry - Textbook Historically, the first chemical definition of an acid and a base was put forward by Svante Arrhenius, a Swedish chemist, in 1884. An Arrhenius acid is a compound that increases the H + ion concentration in aqueous solution. The H + ion is just a bare proton, and it is rather clear that bare protons are not floating around in an aqueous solution. Instead, chemistry has defined thehydronium ion ...
11.1 Describing Chemical Reactions Flashcards | Quizlet 11.1 Describing Chemical Reactions Flashcards | Quizlet Science Engineering Chemical Engineering 11.1 Describing Chemical Reactions 4.7 6 Reviews STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Equation Click card to see definition 👆 A chemical reaction can be concisely represented by a chemical ____ Click again to see term 👆 1/23
Access Free 111 Describing Chemical Reactions Answers Chapter 11: chemical reactions. 1. Deter- mine the correct formulas for all the reac- tants and products 2. Write the skeleton equation 3. Determine the number of atoms of each element in the reactants and products 4. Balance the elements one at a time by using coefficients 5. Check each atom or poly atomic ion to make sure its equal 6.

































0 Response to "45 11.1 describing chemical reactions worksheet answers"
Post a Comment