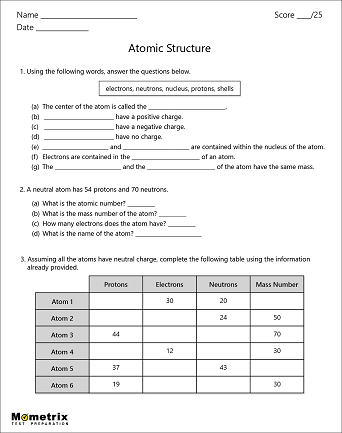
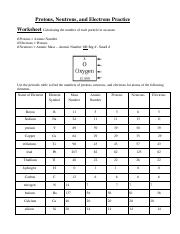
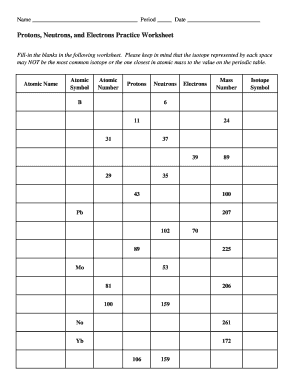
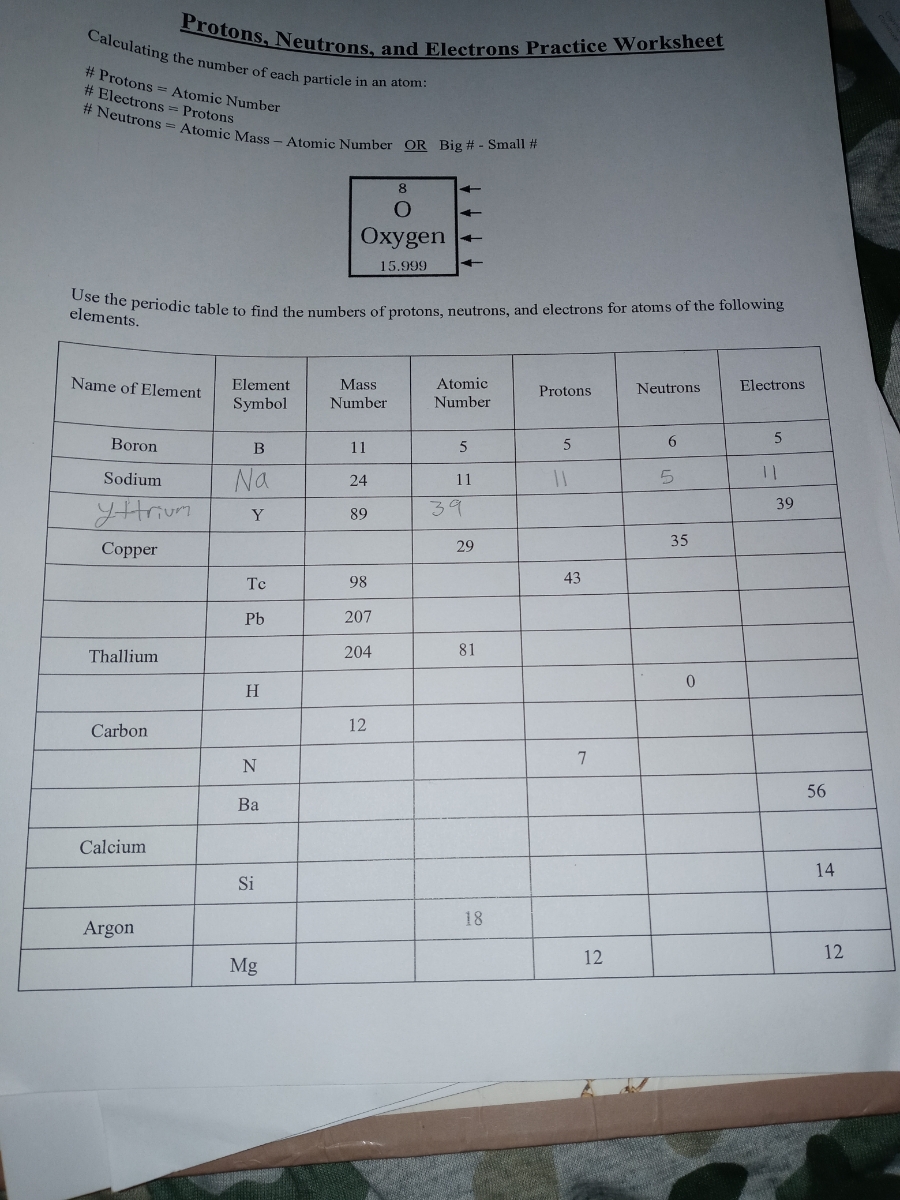
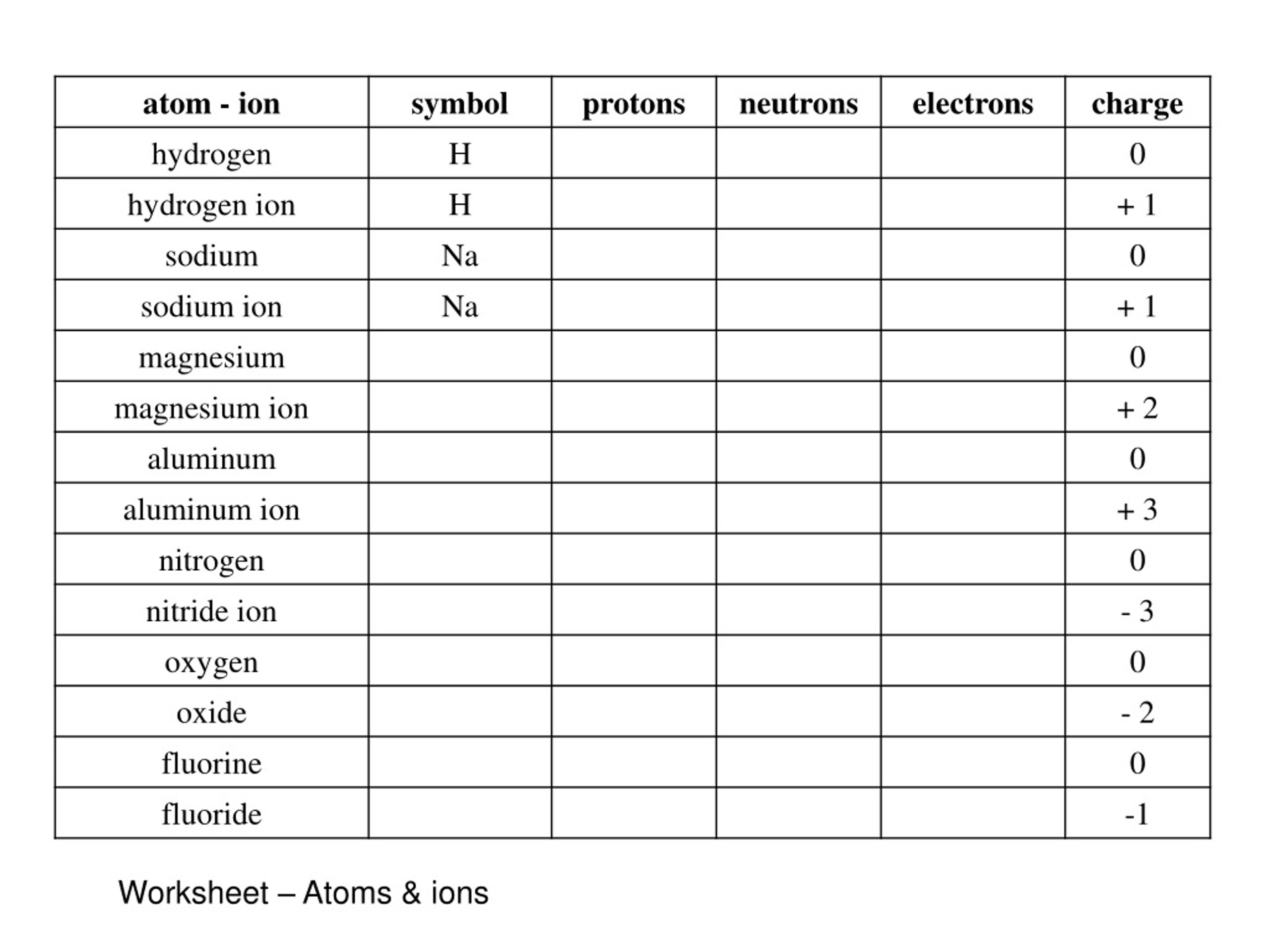
40 protons neutrons and electrons worksheet answers
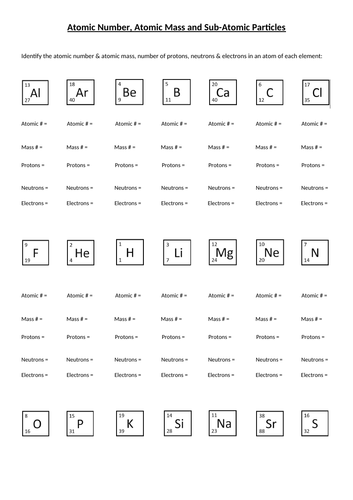
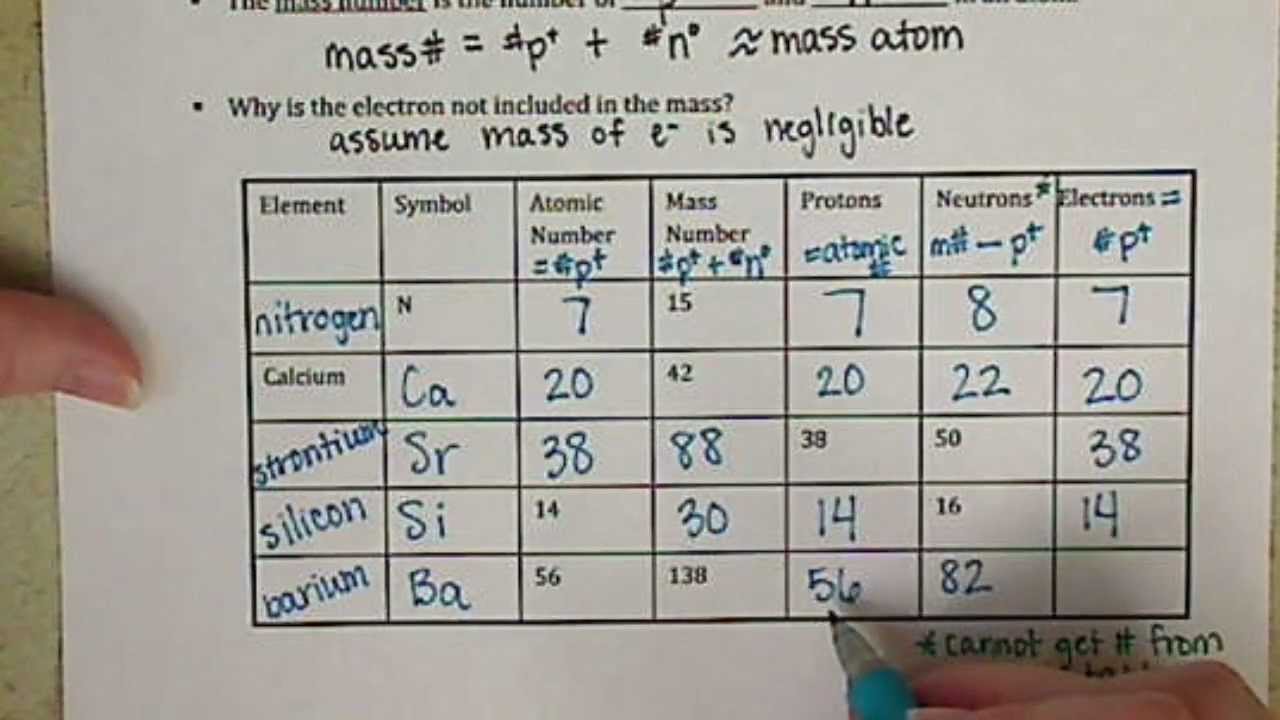
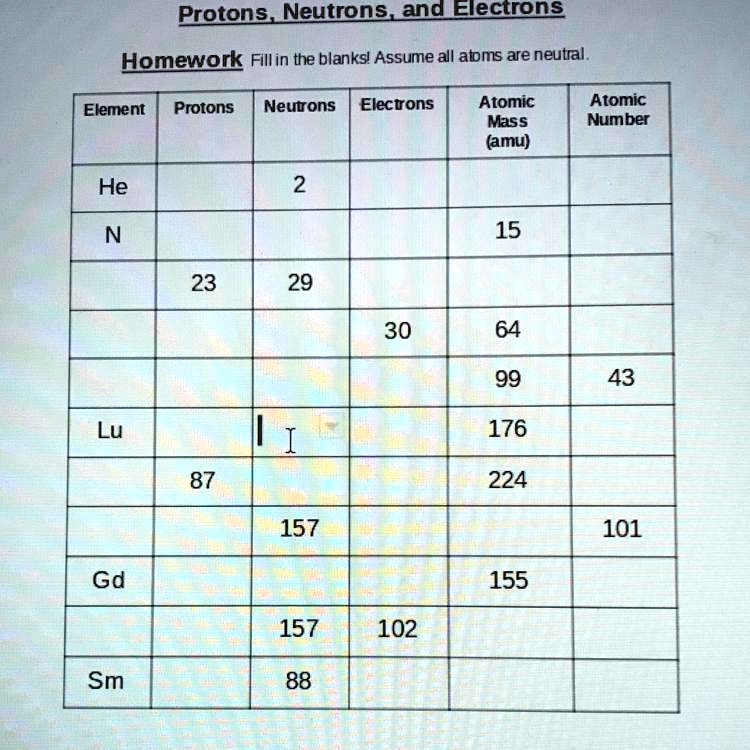
Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 Quiz & Worksheet - Atomic Number and Mass Number | Study.com You will receive your score and answers at the end. ... If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element ...
What is an Atom -Basics for Kids - YouTube Visit for more free science videos for kids.What is an Atom? A good video explaining atomic structure & molecules formation. An a...
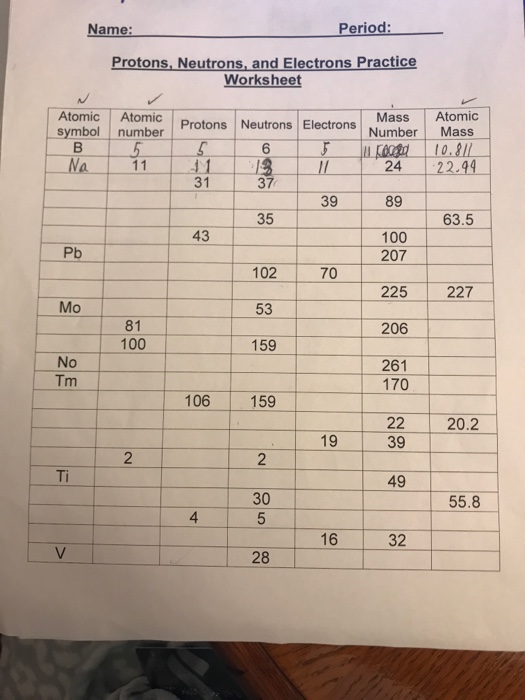
Protons neutrons and electrons worksheet answers
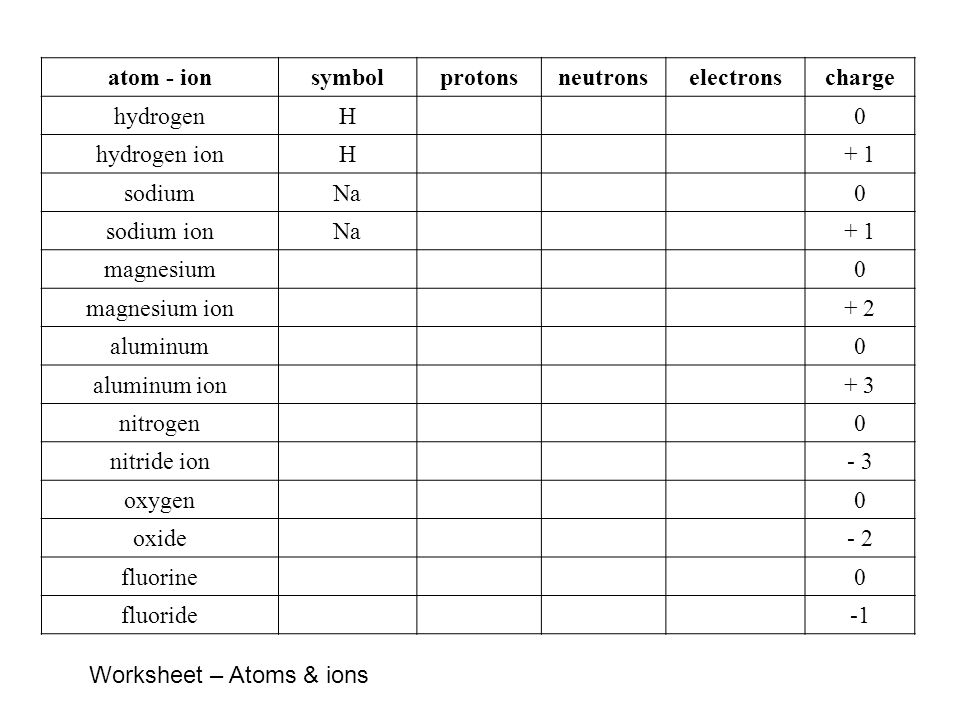
Isotopes Worksheet - Perth Amboy Public Schools PART I. Answer the questions based on the above reading. 1. What is an isotope? Isotopes are versions of the same element. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. The Periodic Table | Chapter 4: The Periodic Table & Bonding ... The majority of the atomic mass is contributed by the protons and neutrons. For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Atomic number - Wikipedia The number of electrons in each element's electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Hence, it is the atomic number alone that determines the chemical properties of an element; and it is for this reason that an element can be defined as consisting of any mixture ...
Protons neutrons and electrons worksheet answers. The Periodic Table & Energy Level Models | Chapter 4: The ... Explain that neon has 10 protons and 10 electrons. There are 2 electrons on the first energy level and 8 electrons on the second level. Beryllium–fluorine Help students fill in the correct number of electrons in the energy levels for the rest of the atoms in period 2. Period 3 Sodium Explain that sodium has 11 protons and 11 electrons. Atomic number - Wikipedia The number of electrons in each element's electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Hence, it is the atomic number alone that determines the chemical properties of an element; and it is for this reason that an element can be defined as consisting of any mixture ... The Periodic Table | Chapter 4: The Periodic Table & Bonding ... The majority of the atomic mass is contributed by the protons and neutrons. For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Isotopes Worksheet - Perth Amboy Public Schools PART I. Answer the questions based on the above reading. 1. What is an isotope? Isotopes are versions of the same element. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons.
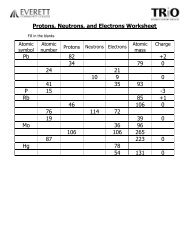
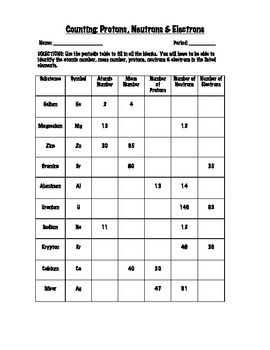
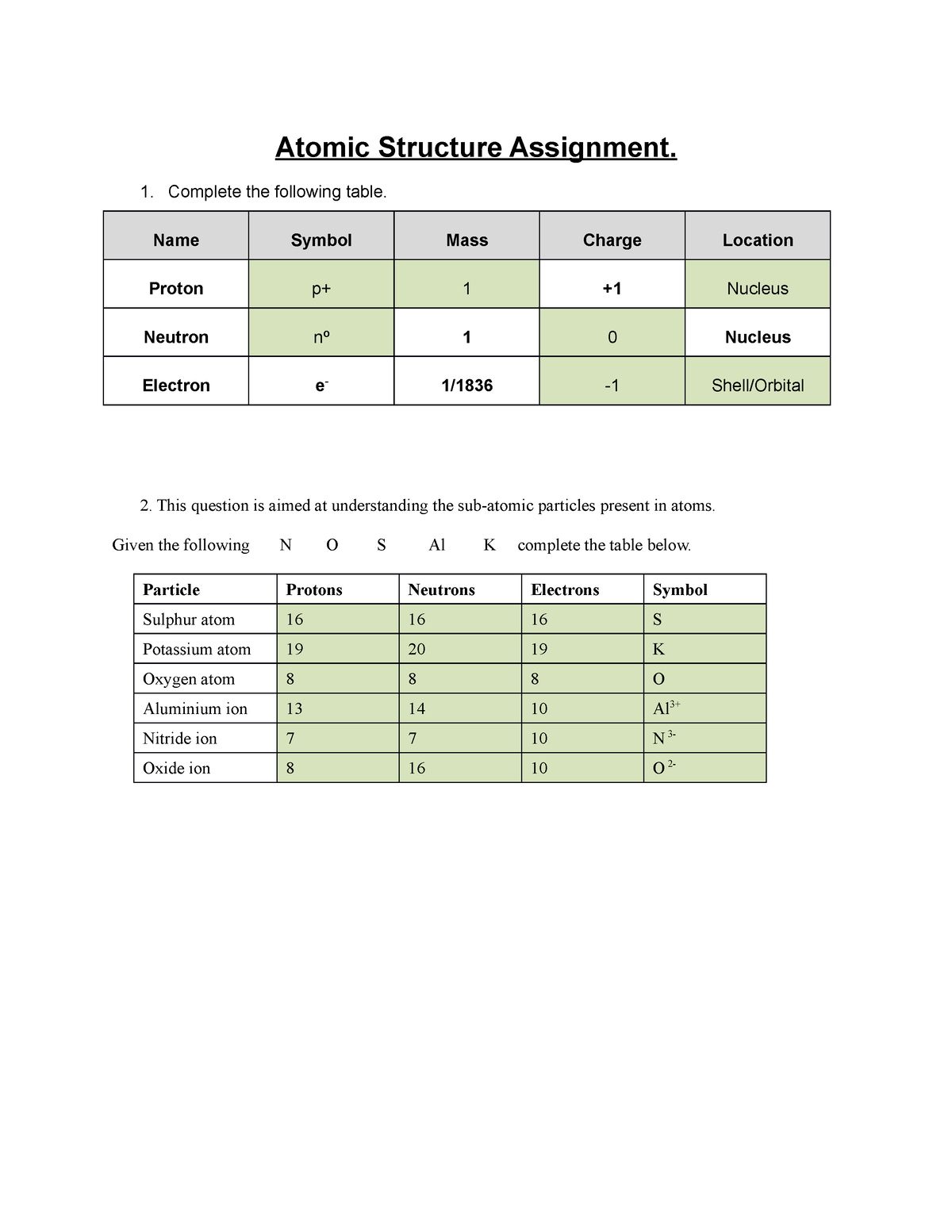










![Protons Neutrons And Electrons Practice Ws [6nq85590eznw]](https://idoc.pub/img/crop/300x300/6nq85590eznw.jpg)










0 Response to "40 protons neutrons and electrons worksheet answers"
Post a Comment